|
|
In this article, I am looking at the human spinal cord. I will show more photomicrographs of tissue samples using the heavy metal impregnation, the Woelke's myelin method, as well as the standard H&E (hematoxylin and eosin) staining technique.
Since the introduction of the famous heavy metal impregnation (1873), other methods got developed that help us to expand our knowledge in medical neuroscience. Because the heavy metal impregnation stains a subset of neurons indiscriminately, it reveals relatively little about the function of an individual cell. Injecting a dye selectively with the help of tiny hypodermic needles allows the study of the anatomy of selected neurons. Another possibility is to utilize the fact that different classes of neurons have chemically different interiors, and hence it is possible to make labeled antibodies that demonstrate some of these differences.
In the following paragraph, I illustrate four different groups of histological methods that are used to investigate the CNS.
These methods were the first ones that allowed researchers to investigate the pattern of branching of axons and dendrites (see [1] for a drawing of a neuron). They are good methods to study the neuronal interconnections. At least two subgroups of heavy metal impregnation methods exist. First, the Golgi silver methods use silver nitrate. They selectively impregnate relatively few cells. These methods are great for outlining the external shape of neurons without revealing much details of the internal cell structure. Second, improved silver methods by Cajal and Bielschowsky are used to demonstrate axons, neurofibrils, and nerve endings (including synapses). A modification by Bodian uses silver proteinate (reduced silver) [8]. It is relatively easy to distinguish the Golgi heavy metal impregnation from a method by Cajal et al. (reduced silver method). If you can see a complete image of a single neuron (clear view of dendritic tree and axon) then you are most likely looking at a thicker section stained with a Golgi method. - I will show a section stained with one of the reduced silver methods by Cajal, Bielschowsky and Bodian.
In an adult human, the spinal cord is about 42 to 45 cm long and about 1 cm in diameter at its widest point. The cord is also segmented. The four main regions or segments of the spinal cord are called cervical, thoracic, lumbar, and sacral. Fig. 1 shows the location of the spinal cord and the four main regions.
In this article, I do not discuss the segmentation of the spinal cord but rather focus on the cord itself, stripped of its dorsal (back) and ventral (front) rootlets. I will show photomicrographs of a cross section (c.s.) from the lumbar region at location L1 (see Fig. 1).
All the shown photomicrographs are taken from prepared mounts sold by Carolina Biological Supply Company [2] and WARD's [9]. With the help of such commercially available mounts, it will not be difficult for the reader to follow my footsteps and explore the interesting world of microscopic anatomy of the central nervous system. - To appreciate these wonderful structures, the reader needs a reasonable microscope. As of today, there is no substitute for looking through the microscope. I cannot stress this point enough. Buying these professionally made mounts is a great reward for every microscopist.
We
start our investigation with the basic layout of a cross section through
the spinal cord (lumbar region, L1). See Fig. 2 for
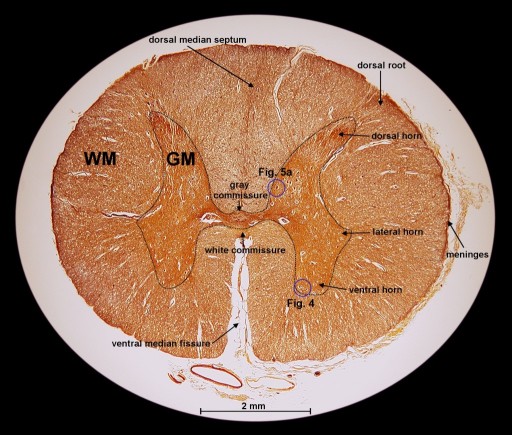
an overview of such a section. We can easily recognize two distinct regions,
the white matter (WM) and the gray matter (GM). I recorded the image for
this figure with a Coolpix 990 camera mounted to a Nikon Eclipse series
scope (see [5] for some details about this setup).
I used a CFI60 Plan Achromat 2x (NA 0.06) with an Achromat swing-out condenser
(NA 0.9/0.22). The top lenses of this condenser were in "swing-out" position
to ensure even illumination. Using PaintShop Pro 7 [6],
I superimposed a line drawing on to the image data. To better understand
the labels in
Fig. 2, I introduce a couple of important
terms frequently used in anatomy (see the following short table).
| Terminology | Explanation |
| Dorsal | Closer to back of body |
| Ventral | Closer to belly of body |
| Posterior | Same as dorsal for bipeds (animals with two legs) |
| Anterior | Same as ventral for bipeds |
| Commissure | Union between corresponding parts |
| Ganglia | Groups of nerve cell bodies outside the CNS |
The
white matter consists of ascending tracts of sensory fibers and descending
motor tracts. The gray matter is regionally specialized. The gray matter
is divided into four basic regions (see Fig. 2), the
intermediate region (includes the lateral horn), the body of the dorsal
horn, the cap of the dorsal horn (substantia gelatinosa), and the ventral
horn region. This separation into different regions makes a lot of sense
when looking at the types of neurons that can be found in each of them.
The following table lists different types of neurons present in the spinal
cord.
| Neuron type | Explanation |
| Motor
neurons |
End directly on muscles, glands, or other neurons in the PNS (peripheral nervous system) ganglia |
| Local
interneurons |
Have all their processes confined to a single small area of the CNS |
| Projection
neurons |
Have long axons connecting different areas |
| Autonomic
neurons |
Innervate muscles and glands |
Over 99% of the all neurons of the human body are interneurons or projection neurons. The cell bodies of large motor neurons are mainly located in the ventral horn region. These neurons supply skeletal muscles. Interneurons and projection neurons can be found in the body and the cap of the dorsal horn. The difference is that the substantia gelatinosa (cap of dorsal horn) contains more finely myelinated and un-myelinated sensory fibers. Autonomic neurons are mainly located in the intermediate region of the gray matter.
After building up a better understanding of the function of the CNS, I then started to look at histology sections with the help of a histology atlas (such as [4]) and a compound microscope. Again, I tried to identify tissue types I am more familiar with.
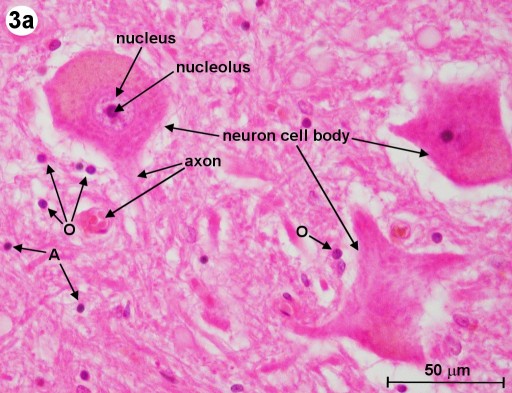
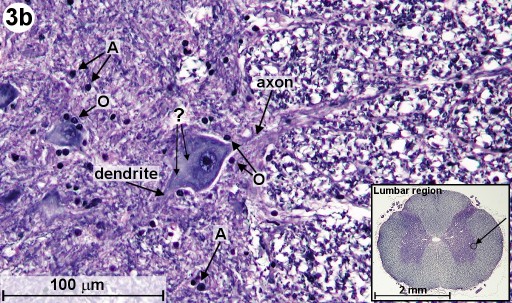
In Fig. 2, we can already see large neural cell bodies located inside the gray matter (GM). To study these large neurons, we look at a section that has been stained with the H&E method and an other one that has been stained with the Myelin method. The H&E staining method usually permits neurons to be readily distinguished from glial cells (neuroglia). (Neuroglia are support cells of the CNS, which surround the neurons.) The Myelin method is used to study the myelin sheaths of axons. In Fig. 3a and 3b, we can see the large motor neurons located in the ventral horn as well as in the lateral horn of the gray matter. (Fig. 3a shows a section from a human spinal cord and 3b shows one from a mammal.) Clearly visible is the cell nucleus and some of the neuron's processes (axons and dendrites). We can also find other cell bodies surrounding these motor neurons. Most of these surrounding cells are neuroglia. The ones closer to the axons are thought to be oligodendrocytes, which are responsible for the elaboration of myelin sheaths around the axons (labeled 'O'). (The equivalent of the oligodendrocytes of the CNS are the Schwann cells of the PNS.) The cell bodies, which are further away from the neurons, are astrocytes (labeled 'A').
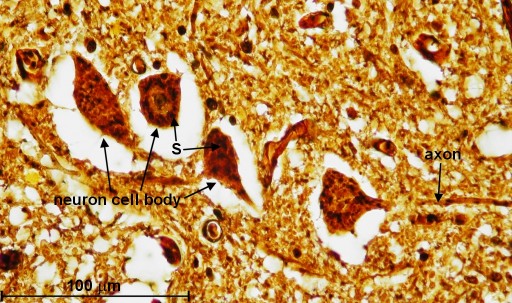
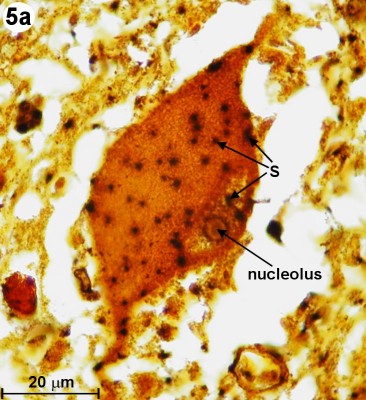
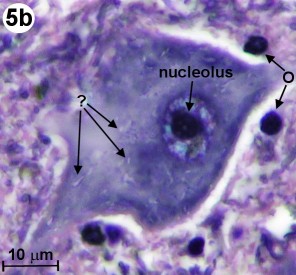
Going back to the silver impregnated tissue sample depicted in Fig. 2, we can see large motor neurons in the ventral horn on the right side (see blue circle labeled "Fig. 4"). In Fig. 4, we are able to identify at least one nucleus and one axon of the motor neurons. The empty spaces around the neuron cell bodies are from the shrinkage due to alcohol fixation. But we can also detect small, black spots inside these neurons. Going back to Fig. 3b (Myelin method used), we can also find small structures inside motor neurons. This time, they appear white (labeled '?'). The black spots shown in Fig. 4 could be silver grains. The question is only, why are they formed inside the motor neurons? - In 1898, Golgi reported a structure inside the neural cell bodies. He called them "internal reticular apparatus". Decades later, the existence of this intracellular structure was confirmed by the use of the electron microscope. Then it became clear that this structure belongs to an important cell organelle, which got later renamed "Golgi apparatus" and eventually "Golgi complex". The Golgi apparatus plays an important role in the intracellular sorting, trafficking, and targeting of proteins. - Now are these dark spots in any way related to the Golgi complex? I do not think so. When looking at Fig. 4, you can see an axon protruding from a neuron cell body (see on right side of image). Inside this axon we can find again these black dots. An electron microscopy image from page 16 in [3] shows a detailed view of an axon. It shows that there is no Golgi complex visible in the axon. But there are mitochondria in the axon region. (Mitochondria are the cells powerhouses. These are organelles in animal and plant cells in which oxidative phosphorylation takes place.) Are these silver grains attached to mitochondria? - Using oil immersion techniques, I studied these spots in more detail (see Fig. 5a and Fig. 5b). The location of the neuron depicted in Fig. 5a is at the edge of the dorsal horn (see Fig. 2 and look for the blue circle labeled "Fig. 5a"). From the size of this neuron, I classify it as a motor neuron although this type of neuron is mainly located in the ventral horn. From this highly magnified image (Fig. 5a), the grainy appearance of these spots is clearly visible. In Fig. 5b, we can clearly recognize the white structures, which are also present inside the axon. At this point in time, I am not sure if the black spots of Fig. 5a enhance the same feature as seen in Fig. 5b. But I am quite certain that in both cases, Fig. 5a and 5b, intracellular features are responsible for the appearance of these tiny structures located inside neurons.
I want to thank Dr. Fei Liu for many suggestions and stimulating discussions. The technical support of C&N for designing the HTML version of this article is greatly acknowledged. Last but not least, I thank all anonymous supporters who provided assistance and equipment.
Comments to the author, Gregor Overney, are welcomed.
| [1] | G. Overney. Exploration of Human Brain Tissue, Micscape Magazine 84 (2002), and references therein. |
| [2] | Carolina Biological Supply Company, 2700 York Road, Burlington, NC 27215 (http://www.carolina.com). Slide 31-3744 (silver method) and slide 31-3738 (H&E). |
| [3] | J. Nolte, The Human Brain, An Introduction to Its Functional Anatomy, 5th edition, Mosby, Inc. St. Louis, Missouri (2002). |
| [4] | B. Young and J. W. Heath. Wheater's Functional Histology, 4th Edition, Churchill Livingstone, London (2001). |
| [5] | Photographic setup is shown at http://pages.prodigy.net/gregor_overney/techniques.html in paragraph "More details about a setup using a phototube". |
| [6] | PaintShop Pro is an inexpensive graphics program for the Windows platforms. It is distributed by JASC Inc. (http://www.jasc.com/). |
| [7] | Background adjustment (or subtraction) can be performed using either the built-in feature of the Nikon Coolpix camera or with the help of image processing software. For instance with Paint Shop Pro 7.04, using the menu "Colors->Adjust->Levels", the user can easily flatten the color spectrum with respect to any RGB color-value. Of course, post-processed background adjustment can reduce the dynamic range per color channel. For 24-bit RGB, the maximum color range per color channel is just 8-bit, or 256 distinct values, per channel. |
| [8] | D. Bodian, Anat. Rec. 69, 153-162 (1937). |
| [9] | WARD's Natural Science Establishment, POB 92912, Rochester, NY 14642 (http://www.wardsci.com). Slide 93 W 3707 (Woelke's myelin method). |
| [10] | A syncytium is a mass of protoplasm with many nuclei but no clear cell boundaries. |
I
used a Nikon Coolpix 990 digital camera with a Nikon MDC relay lens and
an MC-EU1 remote cable. All photomicrographs were image processed. A filter
to sharpen the image was applied and background subtraction was performed
to increase contrast [7]. Text and graphics objects
(such as arrows and dashed lines) were added using Paint Shop Pro 7 from
JASC [6].
| Fig. 1: Simple drawing of the spinal cord with its four main regions: cervical (C1 to C7), thoracic (T1 to T12), lumbar (L1 to L5), and sacral (S1 to S5). (Click on image for larger version.) |
 |
| Fig. 2: Cross section through human spinal cord (lumbar section, L1). The gray and white matter is labeled "GM" and "WM", respectively. The height of this section is 5.8 mm and the width is 7.3 mm. (Click on image for larger version.) |
 |
| Fig. 3a: Cross section through human spinal cord (lumbar region, L1) showing motor neurons. H&E method used. Astrocytes and oligodendrocytes are labeled 'A' and 'O', respectively. The objective magnification used is 40x. (Click on image for larger version.) |
 |
| Fig. 3b: Cross section through mammal spinal cord (lumbar region) showing motor neurons located below lateral horn (see inset). Woelke's myelin method and nuclear fast red stain used. Myelin sheaths are blue and glial cells and nucleoli of neurons appear black. Astrocytes and oligodendrocytes are labeled 'A' and 'O', respectively. White intracellular structures are labeled '?'. The objective magnification used is 40x. (Click on image for larger version.) |
 |
| Fig. 4: Cross section through human spinal cord showing motor neurons located in ventral horn. Reduced silver method used. Silver grains are labeled 'S'. The objective magnification used is 40x. (Click on image for larger version.) |
 |
| Fig. 5a: Cross section through human spinal cord showing close-up view of internal structure of motor neuron located in the body of dorsal horn. Reduced silver method used. Silver grains are labeled 'S'. An oil immersion lens 100x has been used. (Click on image for larger version.) |
 |
| Fig. 5b: Close-up view of internal structure of motor neuron depicted in Fig. 3b. Woelke's myelin method and nuclear fast red stain used. White intracellular structures are labeled '?'. An oil immersion lens 100x has been used. (Click on image for larger version.) |
 |
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape
is the on-line monthly magazine of the Microscopy UK web
site
at Microscopy-UK
WIDTH=1