Microscopic Miracles, a series of tales about small wonders of nature.
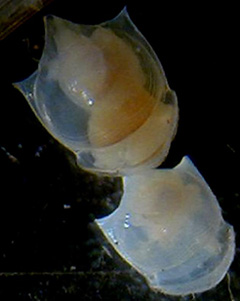
Cavolinia uncinata
Chapter 1 - Pteropods
by Cheryl Gilpin, USA.
Text and images © of the author.

Limacina inflata
|
Microscopic Miracles, a series of tales about small wonders of nature. |
||
 Cavolinia uncinata |
Chapter 1 - Pteropods by Cheryl Gilpin, USA. Text and images © of the author. |
 Limacina inflata |
Imagine you are as beautiful and amazing as a Sea Butterfly.
Imagine weaving your clothes out of carbon by pulling carbon dioxide from the atmosphere and spinning carbonate into the shiniest, most beautiful, and most delicate fabric in the world. Then imagine flying gracefully with wings like a butterfly through the waters of the ocean. Imagine you are able to chew twice your weight in bubble gum and blow a bubble ten times your size. Then when it rains candy, the candy sticks to your bubble. You get a mouth full of treats when you pull the bubble gum back into your mouth. That would probably make you the most wanted snack around. But you would only be a tiny snack. Compared to people, you would be as small as a grain of rice. You and thousands of your friends just like you might even end up making a meal for a whale.
Since your fabric is very heavy you, and many like you will sink. However, as you sink, the bubble that collects your food sometimes works like a balloon to keep you moving up. You can quickly avoid being eaten by drawing in your net and rapidly sinking. When you can no longer labor to stay near the surface you are destined to sink. That becomes a good thing for our Earth. (Read more). Down to the bottom of the sea you will sink, keeping the carbon you hold away from the atmosphere for hundreds maybe even thousands of years. You are replaced by many like you who also end up at the bottom of the sea until one day not so many of you are around to make replacements because the globally inter-connected solution of land, air and water that surround your kind becomes so polluted that you all can't pull the carbon from the atmosphere anymore. Conditions have to be just right for your kind to do this. Not too warm, not too dilute, and not too acidic.
By pulling carbon from the atmosphere and keeping it out, you continue all three of these conditions you need. However, if any one of these requirements is not met, then your ability to pull out carbon slows and that quickly causes the other conditions you need to decrease all over the world at once and then all of the sudden your kind can not exist anymore because we know of no other place in the universe where the conditions you need exist now. Only here, where you are, on Earth.
Now imagine people all over the world who used to not even know of your existence suddenly become more aware. People everywhere on Earth realize that you are very beautiful and amazing. People all over the world think of you as a microscopic miracle. People all realize that they all have a lot in common. Mainly humans all depend on your kind for their own existence. Imagine people also see humankind as a beautiful miracle and suddenly want to stop causing their own extinction. Imagine humans stop polluting so much. Imagine it is easier than people ever thought before and that their lives become better. And so it is. All is well.:-)
Comments to the author Cheryl Gilpin are welcomed.
Special thanks to: Dr. John Wormuth, Department of Oceanography, Texas A&M University, College Station, Texas.
Visit the Micscape Library for other articles in the author's 'Microscopic Miracles' series.
External
resources:
1) See
Science Daily (Feb. 23, 2008). "Small Sea Creatures
May Be The 'Canaries In The Coal Mine' Of Climate Change.”
2) Census
of marine life website
3)
Lalli, C.M.
& Gilmer, R.W. (1989) Pelagic Snails. The Biology of Holoplanktonic Gastropod
Molluscs. Stanford University Press: Stanford, California. 259pp. On Google
Books you
can actually see parts of the text and images of pteropods with their nets extended.
4)
Climate
secrets of marine snail By Helen Briggs Science reporter, BBC News.
Images
The pteropod shells that are round range from 1-2 mm and the long
shells are ca. 0.5 mm wide by 3-4 mm long.
They were photographed with
a stereo-microscope.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sea Butterflies, or Pteropods, are tiny, beautiful marine snails.
Their range extends from the North Pole to the South Pole especially where there is colder water from the deep ocean rising toward the surface (upwelling) and lots of phytoplankton they use for food. They exist in greatest numbers at the poles and have the greatest diversity of species in warm, equatorial waters.
As plankton, they go with the flow of underwater currents. However, they are amazingly efficient at sinking. Through very hard work they flap their wings to keep flying back up toward the surface. Moving themselves up and down in the water column is known as vertical migration.
Pteropods send out a net which slows sinking like a balloon and is used to trap food. These trap tiny phytoplankton (plant-like plankton) and zooplankton(animal-like plankton). Then the food is carried down the net to the pteropod by a conveyor belt system.
In sediments less than 200m deep, shells of pteropods remain for fossil studies and some indicate specific environmental conditions. As their shells form and sink, they play an important role of balancing the carbon dioxide in the atmosphere so that the Earth supports human life.
Unlike most garden variety snails, pteropods have a foot divided into two wings which must be flapped constantly in order to spiral upward toward the ocean’s surface layer. As soon as they quit flapping, they quickly sink from the weight of their shells, which are made of a calcium carbonate mineral called aragonite.
Aragonite is a translucent, fluorescent, strongly birefringent (visible through polarizing sunglass lenses as rainbow-like patterns) orthorhombic (diamond shaped) crystal with irridescent colors. It makes shells that are more beautiful than the calcite form of carbonate but are less stable and require cooler temperatures, higher salinity and a higher pH (lower pH is more acidic). These environmental factors are important to other calcium carbonate depositing organisms; however, pteropods’ requirements for them are in a specific, narrow range. Aragonite formation also depends upon concentration of carbonate ions. Calcium carbonate depositing organisms whose shells dissolve before sinking keep recycling carbonate ions back into the water.
Carbonate ions contribute to pteropod shell formation if the water’s pH is high enough near the surface. In the water, carbonate ions along with other ions also act as a buffer to keep pH from changing. Calcium (bi)carbonate decrease and quit forming when there is too much carbon dioxide dissolved in sea water near the surface. When there is more dissolved carbon dioxide than the ocean’s ions can buffer, the carbon dioxide turns into carbonic acid instead of calcium (bi)carbonate. The water becomes too acidic for organisms to utilize the carbonate ions that still exist in the water for shell formation.
Aragonite shells may account for 50% of the carbon removed from the atmosphere (carbon sequestration). Many pteropods keep removing CO2 from the atmosphere and sinking it to the sediments where the carbon is trapped for hundreds, even thousands of years. Some of the shells dissolve, before reaching the bottom, to recycle carbonate ions back into the water column to help maintain its buffering capacity at the surface. However, neither will happen if the shells are not forming. Even a small change in the balance between air/sea carbon dioxide exchange rates can quickly have huge global impacts. Many interconnected biogeochemical processes have kept that exchange rate stable for thousands of years. However, the increasing amount of carbon dioxide in the atmosphere is destabilizing this biogeochemical system so that the net air/sea carbon dioxide exchange rate which was stable for thousands of years is now starting to change slightly which greatly alters the oceans buffering capacity and the capacity of marine plankton to form shells that sequester carbon. That means a lot more carbon dioxide is going to stay in the atmosphere than does now. That will greatly increase the green house effect that is already causing climate change.
Many scientists now think we are going to lose these organisms in the next 30 years or sooner due to increasing carbon dioxide in the atmosphere and they say that will be catastrophic for marine food webs that support fisheries and whales. Their loss will also eliminate a free natural environmental engineering process provided by these organisms that maintains our own life support system on Earth. See Science Daily, Feb. 23, 2008. "Small Sea Creatures May Be The 'Canaries In The Coal Mine' Of Climate Change.”.
Microscopy UK Front Page
Micscape Magazine
Article
Library
Published in the April 2008 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
with
full mirror at
www.microscopy-uk.net
.