Notes on using a Victorian compressorium on a modern
microscope.
by David Walker, UK.
Keeping live
microorganisms still for visual study or photography can be a potential
challenge for microscopy hobbyists and various approaches have
been recommended over the years. These include:
- Controlling
liquid film thickness: by fixed depth spacers, or dabs of Vaseline
at cover slip corners, or accurate mechanical control with a compressorium.
- Adding
a 'thickening' agent to water, either proprietary or suitable domestic
reagent.
- Adding
a suitable chemical to water to narcotise organism. (See Richard Howey's
article 'Natural Asphxyia')
- Using filamentous
material to limit movement, either artificial (e.g teased micro fibres)
or from the habitat (filamentous algae).
|
Right: Some typical 'critter immobilising' techniques in the
hobbyist's armoury:
1) Dabs of Vaseline at cover slip corners, gently tapping
slip to control depth.
2) Filamentous algae or teased fibres
in sample.
3) and 4) Supports of various thickness,
e.g. aluminium foil or similar thin inert strips for tiniest. 0.4
mm rings (these from NBS) for larger invertebrates.
5) Various
solutions to increase viscosity for the critters. Shown is an NBS
commercial solution, various homebrews reported.
|

|
Like many
hobbyists I've tried most of the above and adopt one or more depending
on the organism type. One approach I haven't tried is the compressorium
which is a mechanical device for accurately controlling the
thickness of the water film. A splendid illustrated survey of designs
both old and more recent has been presented in a Micscape
article 'Compressoria' by Mike Dingley.
Compressoria
can be expensive so have never been fortunate to try one. My brother
Ian though acquired an example as it was included with his splendid
Joseph Casartelli (Manchester) Victorian compound microscope, a
stand described in his illustrated
Micscape article.
For the finest
control of microorganisms especially for the serious worker, a suitable
compressorium is probably one of the best approaches but for more casual
users like myself it's certainly worth deciding if a given design's
advantages outweigh its potential disadvantages coupled with its cost.
Especially the older designs which may not always ideally suit the modern
microscope. The pros and cons may depend on the exact design but for
the Victorian design tried, some observations from the author's experiences
are offered below.
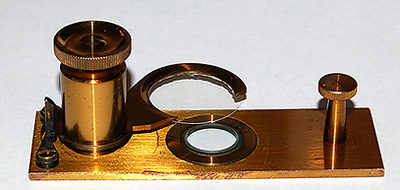
A beautifully
made lacquered brass compressorium supplied as part of a Joseph
Casartelli microscope ca. late 1850's. It has the same footprint
as a 3x1 inch slide. In this design the upper slip remains
parallel to, and moves vertically with respect to, the glass base.
The screw is fine enough for precise control of water film thickness
and slip support can swing out for preparing a sample.
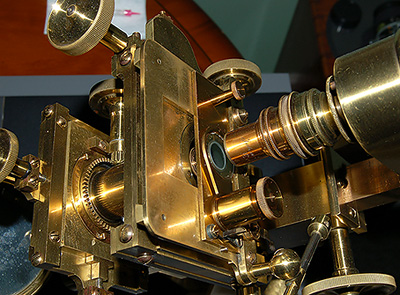
On
my brother's Casartelli stand with single objective nosepiece it
works well (and looks fantastic!). Microscopes of this vintage often
used objectives as a condenser, as here, which because of its narrow
front housing can also focus into the recess.

On
a more modern stand like a Zeiss shown above, the raised pillars can
prevent ready use of different objectives. A six turret nosepiece,
rather than a five shown here, may also encroach on objectives either
side, bulky objectives may also be a problem.
Depopulating the nosepiece somewhat can
help but the pillar
height meant that the objectives had to be set very high to clear
these pillars before swinging in a second objective to refocus. This may not be
a major inconvenience on many stands
with lightly loaded rapid action coarse focus, but on the Zeiss Photomicroscope
III with heavily loaded coarse focus I found this tedious.
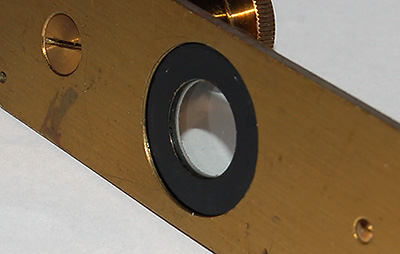
The
lower glass plate is recessed by about 2 mm preventing accurate
setting up of Köhler with certain condensers like the Zeiss achromatic-aplanatic
phase / darkfield condenser.
If the condenser focus is set high to focus in the recess, moving the
compressorium with stage controls could scratch the condenser top on edge of black
ring.
A long working
distance condenser circumvented this but then lost some
facilities like darkfield or full immersion work.
A number
of organisms studied also tended not to adopt a relaxed pose when compressed
to immobilise e.g stentor just contracted. So preferred giving them
more space attached to algae etc on a conventional microscope slide
with coverslip where
they adopted natural feeding poses (and the algae 'set' the film thickness). This observation may reflect my
lack of patience waiting for critters to adopt selected poses under
a compressorium but found it easier with other methods.
The
precise control of film thickness with a compressorium was certainly more
convenient compared
with the more hit and miss approach with e.g. Vaseline at cover slip corners
(sometimes to the organism's demise).
But overall,
for my preferred way of looking at critters, flitting from high to low
objectives and trying multiple lighting techniques, I found this design
more of a hindrance than a bonus. If looking out for a compressorium
design, two key
features that may have bearing on its ease of use is height of any fittings
and if the bottom plate design can accommodate the focus of the condenser
to be used.
Comments to
the author
David
Walker
are welcomed.
Footnote:
'NBS' mentioned above was Northern Biological Supplies a UK company formerly
run by the late and sadly missed Eric Marson. Other commercial reagents are
available.
Acknowledgement:
Thank you to my brother Ian Walker for the loan of the compressorium
and permission to share an image of the Joseph Casartelli microscope.
Microscopy UK Front Page
Micscape Magazine
Article
Library
©
Microscopy UK or their contributors.
Published
in the April 2008 edition of Micscape.
Please
report any Web problems or offer general comments to
the
Micscape
Editor
.
Micscape
is the on-line monthly magazine of the Microscopy UK web
site at
Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
with
full mirror at
www.microscopy-uk.net
.




