|
Another Gallery of Polarized Crystals by Richard L. Howey, Wyoming, USA |
Virtually every amateur microscopist has a story about the one that got away and I’m not talking about fish here. Most of us have passed up a bargain on a microscope or major accessory and later regretted it. Of course the other side of that coin is when we have yielded to temptation and impulse and purchased something which did not live up to our expectations. Then, there are those unfortunately rare occasions when according to your astrologer, horologist (and, no, that’s not a dirty word), and your banker everything aligns and says: Full Speed Ahead! About 8 months ago, I had one of these special opportunities and as Oscar Wilde said: “I can resist anything but temptation.” On offer was an older model Zeiss polarizing microscope with both a monocular and trinocular head, a circular, rotating, calibrated stage, a condenser which I am still figuring out, 3 rotating calibrated compensators, 4 slide compensators, 4 filters, an epi-illuminator in addition to the usual transmitted one, and a long tubular compensator which I suspect has something to do with epi-polarization (but, I don’t yet know), and a set of 3 rectified lenses and a condenser (which a late colleague of mine had ordered) which were included in the package gratis because the dealer had known and admired my colleague and knew that we were friends. Everything is in pristine condition, no delamination and all of the moving parts move exactly as they were designed to–smoothly and precisely. In other words, this was an instrument that only a village idiot would pass up. My banker advised me to sell all of my Studebaker stock and proceed. The cost was less than the annual budget for cannonballs in Andorra. The last I read was they still only have 2 cannons.
The test of any instrument is, of course, its performance. I much prefer older, quality instruments for scientific, economic, and aesthetic reasons. The same holds true for digital cameras as well as microscopes for me. I am still quite happy with my Nikon CoolPix 995, quaint as that may seem. So, what you’re going to get here is a series of polarized images from my Zeiss, taken with my CoolPix 995 and processed with my old, comfortable computer graphics software PhotoImpact XL–a lovely, antiquated combination for an unlovely curmudgeon. So, remember that the following images are constrained by antiquity–both the instruments’ and mine, for I still have so much to learn about achieving peak performance.
Now, there are all kinds of things to examine with polarized light: thin sections of minerals, of bone and scalp, muscle tissue, Paramecia, mosquito larvae, plant sections, hairs, fibers, crystals in plant leaves, and the list goes on. So, here’s a whole bunch of topics for future essays but, in this one, I want to focus on some slides of crystals which I made up some years ago and stored in slide boxes for future reference. The ones that didn’t produce interesting results, I discarded and the other ones I labeled carefully so I could make fresh slides using the same combinations. Some of these slides are over a decade old and I’m lazy so, you’re going to get images from some well-aged slides. Inevitably, some of them have attracted some dust, but in some instances, a good blast of canned air will suffice. In other cases, it may be necessary to use a pair of micro-forceps with your stereo-microscope to remove fibers. In a few instances, the crystals seem to have degraded and are no longer of much visual interest. Occasionally, a drop of 70% isopropyl alcohol will dissolve the crystals and cause them to recrystallize in interesting ways but, there is certainly no guarantee.
If you are going to store such slides, it is essential to try to keep them as dust-free as possible. Any type of slide that is in a mounting medium needs to be stored flat in a horizontal position so that the mountant does not run. With crystal slides, I do not recommend trying to store any that contain substances that are deliquescent (hydrophilic) because they will be unstable and small pools of liquid can persist in various areas on the surface of the slide even after years. A couple of examples are Rochelle salts (Potassium sodium tartrate) and Magnesium chloride.
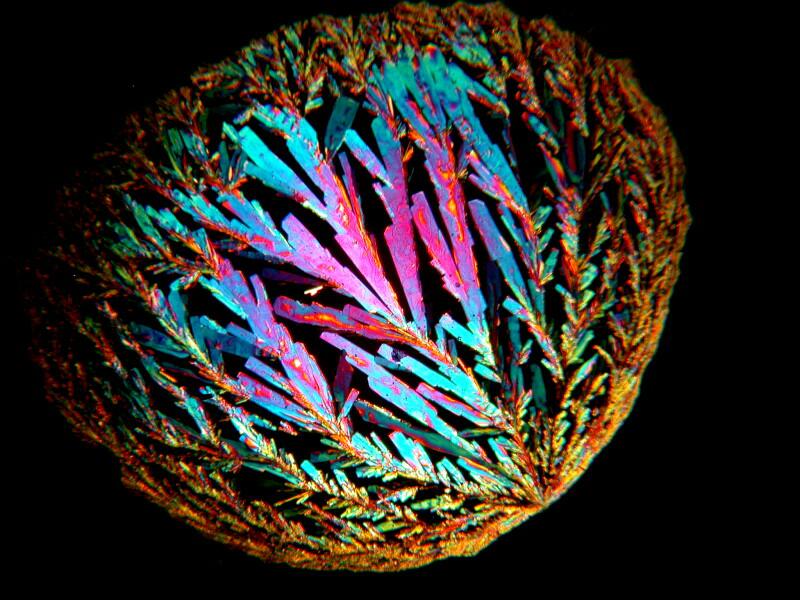
The very first slide which I examined with this new/old instrument was one that had only 3 little clusters of Ascorbic acid on it and I’m going to show you one which was taken using a rotating quartz compensator.

This slide dates from 2005. The one next to it is a year later and uses a fresh solution of Ascorbic acid. The differences are striking.
Ascorbic acid is one of those wonderful substances that mixes in interesting and sometimes dramatic ways with other reagents and can produce a dazzling variety of forms and yet, there are often patterns that signal to you and you say–ah, there’s some Ascorbic acid somewhere in this mixture. (In preparing such slides, one must be careful, however, because sometimes excessive heat can produce toxic by-products with some chemicals.)
Not long ago, I was looking at the marvelous book of W. A. Bentley full of pictures of snowflakes and now, I have gotten curious about what might happen if I were to put some slides with various chemical mixtures in the freezer compartment of our refrigerator. (Please don’t tell my wife.) For starters, I think I’ll put the slides in those little plastic slide shipping boxes that hold 5 slides. On frosty mornings, when I see the lovely patterns on the window panes in my study, I wonder–what would that look like if it weren’t water, but a solution of Ascorbic acid or Copper sulfate or Citric acid?–and this list goes on and on.
One day when I was feeling especially creative (in other words, I didn’t have a clue what I was doing) I made up a slide which was a mixture of Ascorbic acid, Metformin ( a diabetes medication), and Vanillin (a crystalline vanilla extract which comes from an orchid)–and I hadn’t even had a single sip of single malt or anything else. Here is the result:

Circles and wheels often appear on Ascorbic acid slides sometimes accompanied by a variant of a Maltese cross as in the example below.

Tincture of iodine which tends to produce interesting geometric crystals when combined with Ascorbic acid can produce a tiny Euclidean circus.

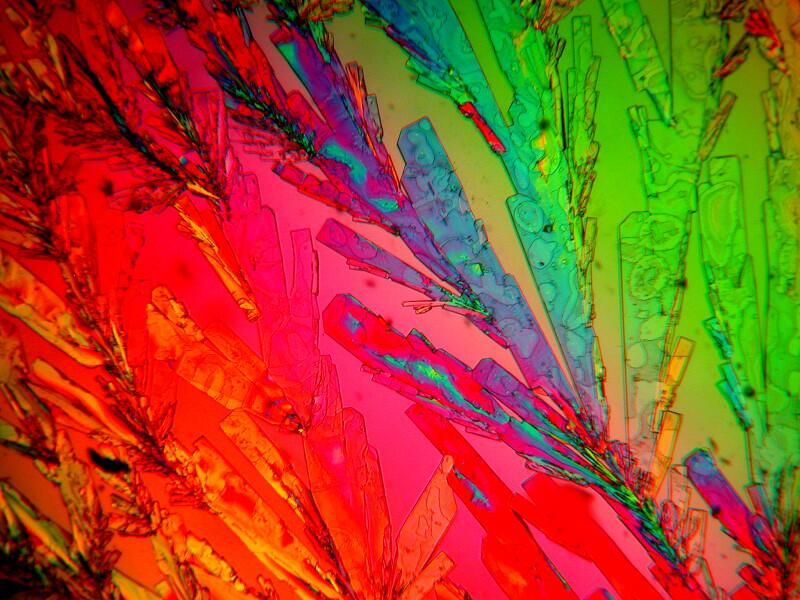
Metformin can, when mixed with Ascorbic acid and some other chemicals, produce some splendidly colorful results. Does your daughter or granddaughter have a microscopic doll house? Well then, mix some Ascorbic acid, Metformin, and Vanillin and create a micro “rag-rug” for her mini-recreation room.

When I was a child and I got a cut or a scrape, if we were out of iodine, my mother would use Mercurochrome to which I am sure the manufacturers added Eosin Y. I distinctly recall that from a certain angle the liquid in the bottle appeared light orange but, from another angle, it would appear a pale fluorescent green and that is a distinctive characteristic of Eosin Y. Now, however, when I buy Mercurochrome for my crystal play sessions, it is colorless and that is expressly stated on the bottle. I have no idea why such a measure was taken but, I am sure it had to do with marketing and money. I’m not particularly concerned, because for making crystals the colorless version produces some fine results.
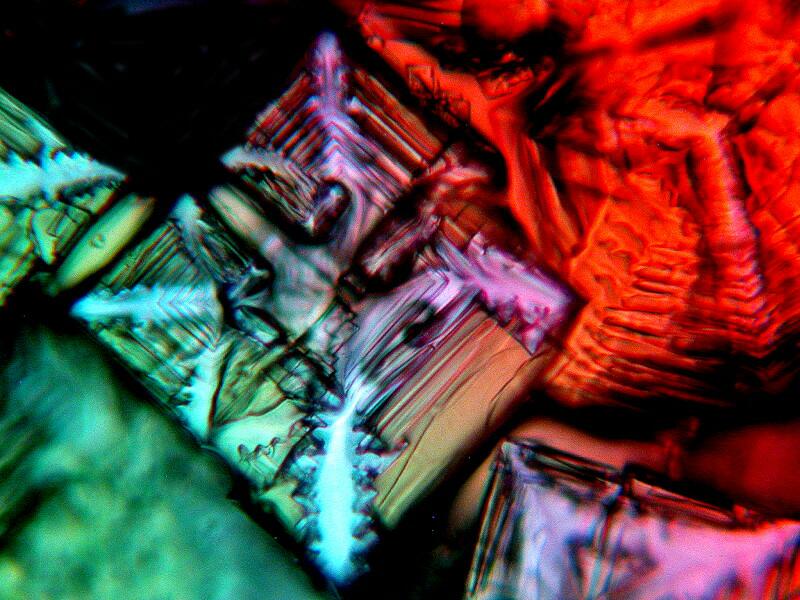
Out of my recent efforts, a mixture of Mercurochrome, Ascorbic acid, and Metformin produced my favorite results. Below is an example.
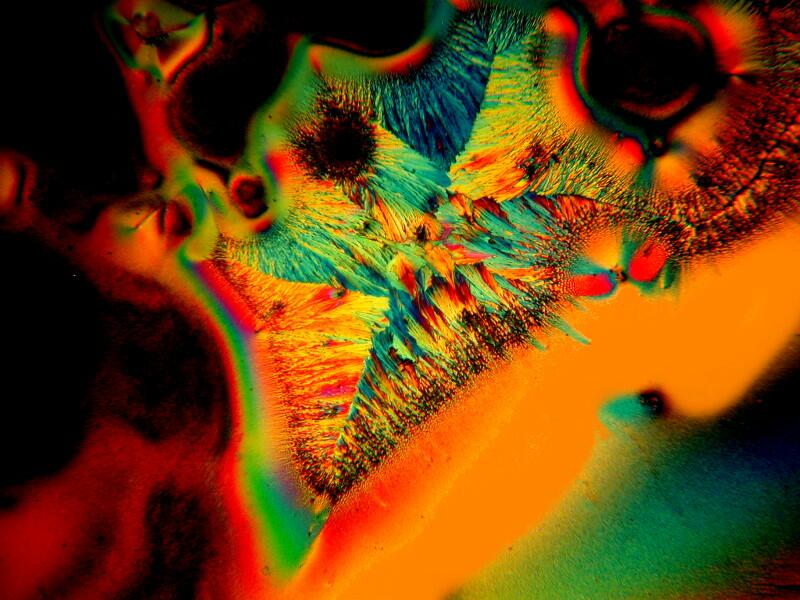
At a flea market–I was terribly disappointed; there wasn’t a single booth that had any fleas for sale–I found a small bottle of a liquid called Sanafiltil which is used for treating fungus–on toenails and that sort of thing, I assume. In any case, a bit of it mixed with Ascorbic acid can produce some wonderfully intricate branching patterns and I’ll show you 3 examples below.

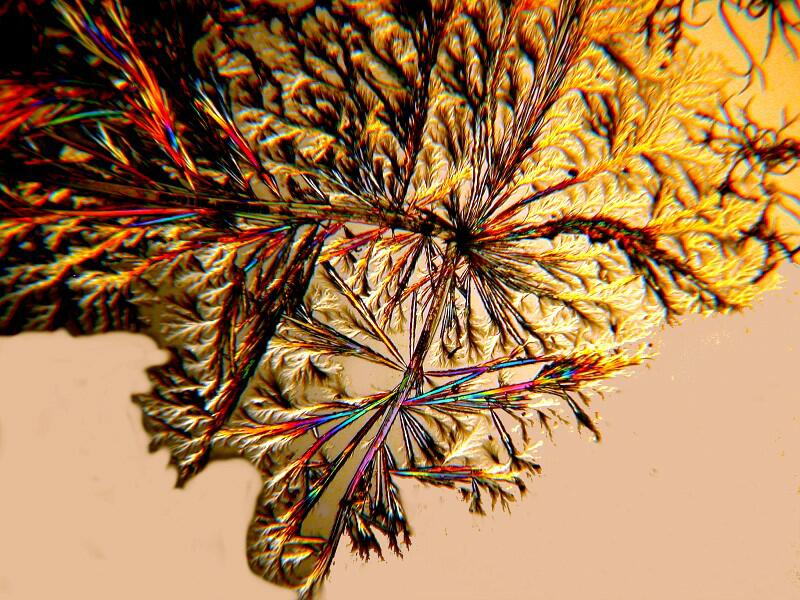

If you prefer waves to branches, a little Tartaric acid mixed with some Metformin will produce the desired result.

Sometimes the judicious addition of a bit of a biological stain to a mixture can produce some unexpected results. Methylene Blue is a common and useful laboratory stain and when mixed with Metformin and Ascorbic acid can create surprisingly different patterns. Below are 3 images of this mixture.
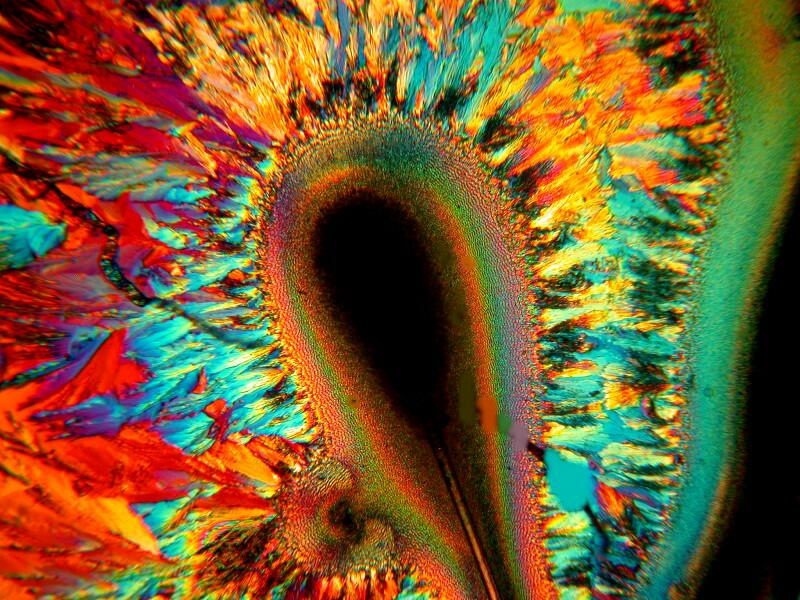
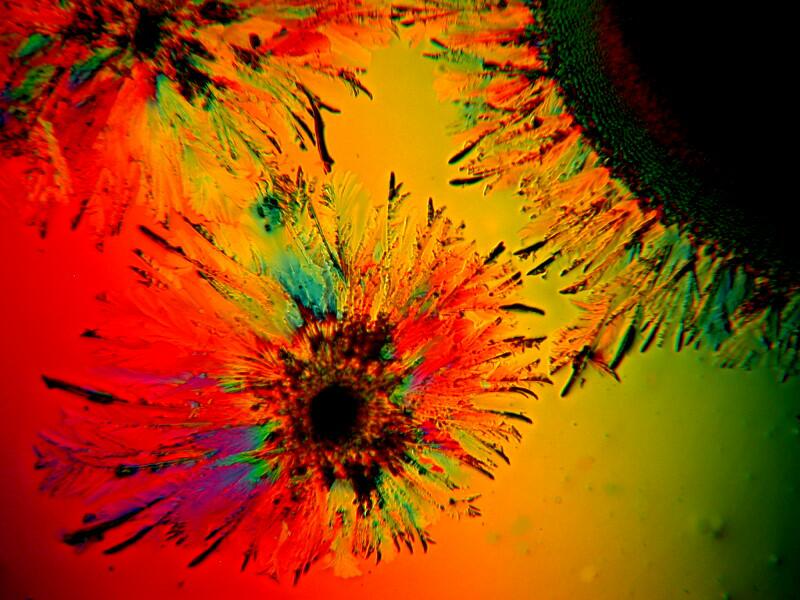

What these, and many other examples of crystalline mixtures demonstrate is that it is extraordinarily difficult to anticipate or predict what might appear on your slides. With certain substances, such as Ascorbic acid, there can be certain patterns which appear and reappear and which one can come to recognize but, nonetheless, there remains a high level of unpredictability with other compounds.
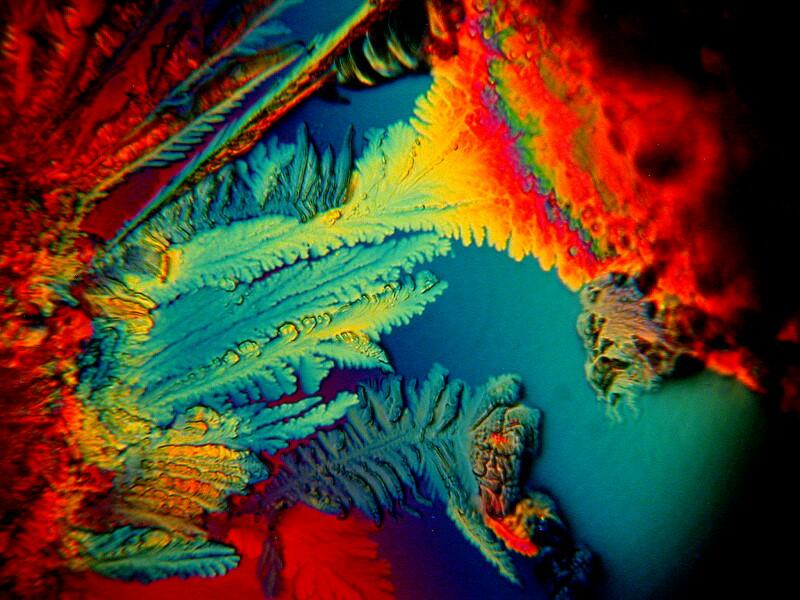
On another one of my “creative” rampages, I mixed the following: Ascorbic acid, Stevia (the non-sucrose sweetener derived from a semi-tropical bush), Fluoxetine (an anti-depressant), and the laboratory stain Neutral Red (just in case I needed some extra colors). The 2 images below are from the same slide and yet strikingly different. This is an issue which I intend to follow up in another essay which will also show how very subtle changes in the positioning of compensators can also significantly alter the appearance of a specimen photographed at exactly the same position.

Finally, another “old reliable” when it comes to biological stain and polarization is Orange G. The two images I am going to show you are just the stain unmixed with any other substance. The second is a close-up of the first. This dye, fairly typically, manifests rods and clusters of rods and sometimes acicular (needle-like) crystals.
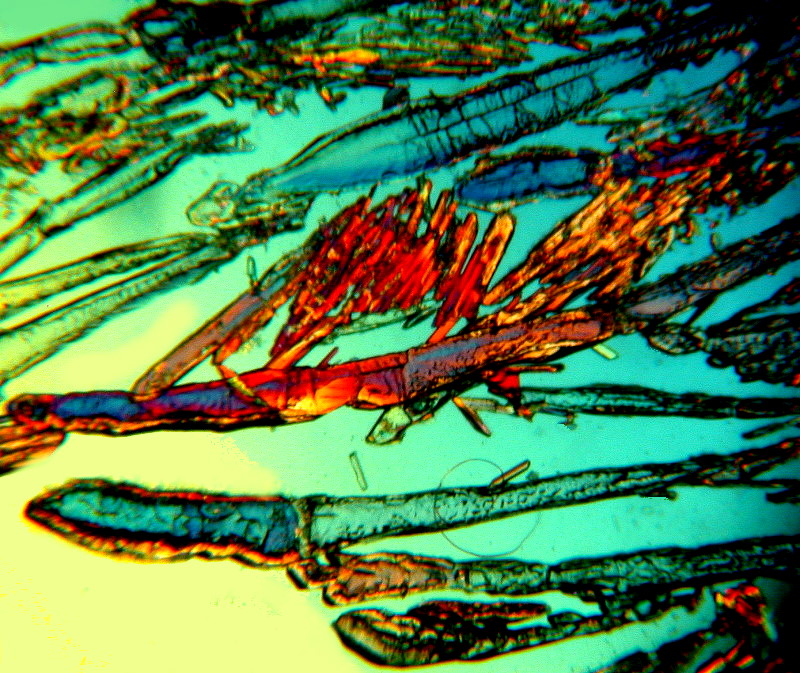
And a close-up:
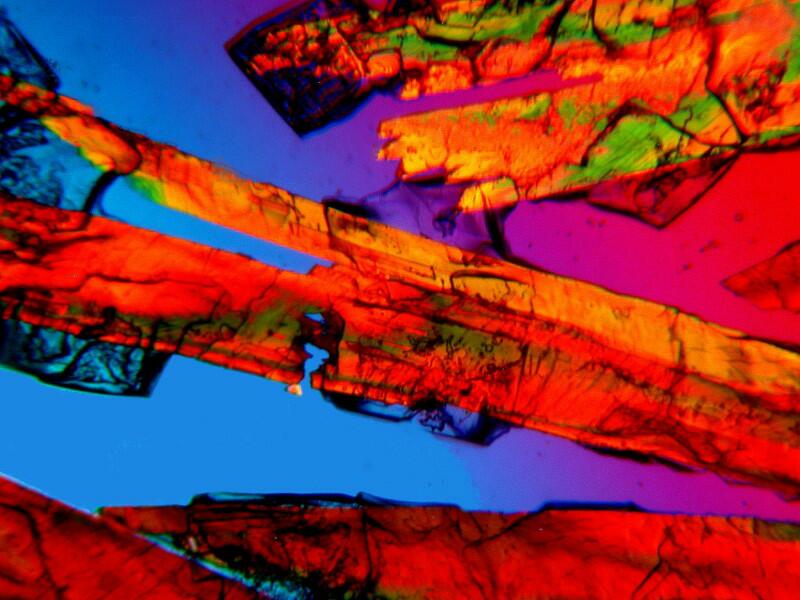
I hope you have enjoyed this brief tour of a tiny facet of micro-art and I hope that as I continue to investigate examples of different types of specimens with this new/old microscope, I will find the opportunity to explore more aspects of the aesthetic dimensions of crystal mixtures.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the April 2013 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .