My Route to Microscopy and Crystal Pictures
Theo Wyatt, UK
It
is, I guess, a reflection on the narrowness of my grammar school
education, that I had never looked down a microscope until some date
in the early 1950's. Then, in a chance meeting with my younger
brother, with a newly acquired degree in zoology, on his way with his
own microscope to his first job as entomologist in Trinidad, he
showed me cheese mites, house dust mites and tardigrades. I was
intrigued but not captivated.
In 1961 I spotted in a local junk shop a pre-war German toy microscope with a magnification of about x50, and bought it as an educational present for my eldest daughter, then aged 8. Naturally I had to demonstrate how it worked. On the mantelpiece of the girls' play room was a large jar which had once held pickled onions but was now home to a population of sticklebacks, brought back from a weekend at Salisbury with my sister's family. I scraped a little of the green algae coating the inside of the jar and put it on a slide under the microscope. There were two wondrous transparent creatures, brightly illuminated, with two mouths where their head should have been, each surrounded by a rotating windmill of cilia. They were rotifers, found in standing water everywhere; and I was completely hooked. I had to get a real microscope for myself.
I bought Exchange & Mart each week, and there a few weeks later I spotted “For sale by tender: three microscopes. Lambeth Group Hospital Management Committee”. I walked over to Lambeth Hospital in my lunch hour. Two of the instruments on display were just what I thought I wanted – simple black Leitz machines from the 1930s. I thought I had a reasonable chance of getting one of them if I bid £25 for each. The third was a museum piece in shining brass, bristling with massive knurled knobs, some performing functions I could not discern. I was frankly frightened of it, and feared I should never be able to learn how to control it. I nevertheless risked a £10 bid, and a week later it was mine. It was a Watson Edinburgh stand, introduced in the 1890s. I subsequently owned more modern and more sophisticated microscopes, but I spent more hours with and got more pleasure from that old brass instrument than any other. It was also the subject of a truly remarkable coincidence.
We had joined the Merton Scientific Society and there had become friendly with Arthur and Hilda Wyatt. Arthur – no relation – some ten years older than us, was a retired hospital technician and a passionate fossil hunter, whose collection was donated by his widow to the Horniman Museum and is on display there. After one Scientific Society meeting Arthur and Hilda came round to our house and were ushered into the music room where the old microscope happened to be out on a coffee table. As soon as he entered the room Arthur cried out “How on earth did you come to have my old microscope?” It was indeed the very instrument he had used many years before on his first job at Lambeth Hospital.
Arthur
gave me a lot of useful advice on microscope technique and I still
have a box of specimens he had mounted, but it was another contact
made through the Scientific Society who converted microscopy from a
passive spectator sport of rotifer-watching into an absorbing
creative hobby.
Eric Simpson
He was the brother of Sir Keith Simpson, a forensic pathologist who came to national prominence in several spectacular murder trials, the most noteworthy of which led to the conviction, largely on Keith Simpson's evidence, of John Haigh, who dissolved the bodies of his victims in sulphuric acid, in the erroneous belief that the absence of a body would make conviction impossible.
Eric was the nearest thing to a polymath that I ever encountered; there seemed to be no subject on which he could not provide convincing information. He showed me how to make crystal pictures for the microscope, a skill which he had used, when briefly unemployed in the Depression, to make slides for sale to the microscope shops in London. His last employment was in charge of laboratory equipment at Charing Cross Hospital.
He was also an obsessive hoarder, as we discovered when, after the death of his wife, he left Wimbledon to live near his son on the South Coast. His son asked if we would help to clear his garage before the house was put on the market. The garage was at the bottom of the garden, accessible from the road by a rough track. The door from the garden opened on to a winding canyon, just wide enough for human passage, leading to the centre of the garage, between walls of boxes and cases stretching to the roof. It did not take us long to discover that most of it was rubbish. There was a large tin full of used scalpel blades, many of them blood-stained; there was an even larger tin full of irreparably broken artery forceps; there were several broken X-ray tubes. We hired a skip from a waste-disposal firm, had it placed in the track outside the garage, and started filling it. We had however to be careful, because among the rubbish were some treasures. There were four working microscopes. Three of them were brass instruments contemporary with my Watson. I sold these for him to a dealer through Exchange & Mart having spent several hours putting them as best I could into good working order by cleaning, lubricating and adjusting the moving parts and disassembling the objectives and eyepieces and cleaning the tiny internal lenses. When the buyer came to collect them I asked him what sort of customer would be buying them and for what purpose. “Oh” he said, ”none of these is ever going to be used. They will all go to smart interior decorators who will put them as conversation pieces in illuminated alcoves in expensive penthouses.”
The fourth microscope was an apparently unused Zeiss stand in its box. I bought it for myself because it incorporated a rotating stage, a facility which I had not seen on any other stand and which I fancied might be very useful in making crystal pictures, as indeed it proved to be.
More exciting to me than the microscopes were the cardboard boxes containing more than 100 little bottles and tubes, all meticulously labelled, containing samples of chemicals, some familiar like DDT, some so esoteric that I could not find them in the large chemical dictionary I had inherited from my years in the Chemicals Division of the Board of Trade. Eric had said that he liked to keep samples of all the materials that passed through his hands, and here they were waiting for me to experiment with.
Crystal
pictures
For a year or two I spent a lot of time in the absorbing creation of crystal pictures, trying one chemical after another, carefully scanning the resulting slide for any element of pictorial interest. I slowly developed a clearer concept of what I wanted to produce – a picture with visual impact; a picture with a satisfying feeling of composition; a picture which was more than a random assembly of vivid colours evoking wonder solely because it was produced from a colourless substance; a picture, in short, that an owner might think worth framing and hanging on the living room wall. I found that these requirements seemed most often to be met if the slide contained shapes which could be related to shapes in the natural world.
Equipment
It will be as well if I describe here the equipment I used. All the microscopes I acquired came with simple achromat objectives, and I used the lower powers of these for all my early slides; but as my standards rose, the out-of-focus corners became unacceptable, and I invested in a Russian x3 plan-achromat, which I used exclusively for crystals thereafter. The mechanical stage of the Watson, which had so frightened me in Lambeth Hospital, proved indispensable in the systematic examination of a newly created slide; the combination of mechanical with a rotating stage, which I enjoyed with the Zeiss stand, will, I suspect, be no more than a pipe-dream for most would-be crystal picture makers.
I had a Pentax SLR camera body with a screw thread, for which I had to buy a connector to attach the camera to the microscope. Between camera and microscope I fitted a bellows attachment, of the type used for macrophotography, which enabled me, easily and accurately to resize the image to fit the frame.
Enhancing
the Image
When the crystal is left to its own devices, the image it produces often contains just too much vivid colour; the eye cries out for light and shade. If you can rotate the slide, each block of colour changes through the other colours of the spectrum and through white and black. There may well be, among all these alternatives, one which is a dramatic improvement on what you started with.
If the crystal does not cover the whole slide, you will need to fill the empty areas with some background colour. The simplest method is to take a sheet of cellophane wrapping from (say) a cigarette packet, and insert it between condenser and slide. You may need to experiment with more than one thickness and by rotating the sheet. When you find a formula that works, you may think it worth incorporating it in a card mount that can be easily manipulated when required in future. And note that whatever colour you add to the background will be added to the whole slide.
Maverick
Crystals
Some chemicals can take you by surprise. We all know that a naphthalene mothball, shut up in a drawer full of socks, will eventually disappear completely. The molecules on its surface, when in contact with air can sublime, changing from solid to gas without any intervening liquid phase. Some of those in Eric Simpson's little bottles did the same. One was styryl methyl ketone. It produced quite interesting and colourful slides, but if you put them away and looked at them a fortnight later, the slide would be empty; but if you left them for just a few days you would find a totally transformed image. The molecules at the edge of the cover slip, being in contact with air, had wandered off into the wider world. Those further from the open air, now having more elbow room, shuffled around in search of fellow molecules to which they could bind, and in doing so formed large blocks of uniform colour. The result suggested to me the title Recumbent Figure with Guitar.
P-chlorphenyl glyceryl ether melted easily but crystallised extremely slowly. Having made the slide and waited 15 minutes in vain for a result to appear, I abandoned it as a failure. Taking up the empty slide a few days later to try another chemical, I was astonished to find the picture I have called Satellite. A week later the same slide produced the picture I call Degas' Ballerinas.
Salicin
melted
readily, but in its liquid form was so viscous that it refused to
flow by capillary attraction between the slide and cover slip,
forming a rope of material which pushed the glass surfaces apart. The
result was an intriguing picture, but not one that anyone would want
to hang on the wall. If there had been a next time I would have tried
to find a solvent.
Unanswered Questions
Let me imagine the first one: “How on earth do I get hold of a sample of benzoyl eugenol?“ Brutally frank answer; “Don't even try; it is impossible.”
Sour grapes answer: “If you succeeded, you would be very unlikely to replicate my result; much depends on the impurities in the particular specimen.”
Philosophical answer: “In this area, chance is king. My brief friendship,
half a century ago, with Eric Simpson, and the invitation to help clear his
garage, were two outrageous pieces of luck; without either I would never have
made a single picture. But they would never have happened if I had not paved the
way by joining the local Scientific Society. You have already taken the first
step on that path by consulting the website of Microscopy-UK. Keep in touch
with fellow enthusiasts; chat up any doctors or hospital lab technicians you
meet socially; ask the pharmacist at your local chemist if he ever disposes of
drugs from which he could spare a few granules for you to experiment with.
Reflections
I do think that, given the care and skill that must be devoted to locating an image in a crystal slide and manipulating it into something of aesthetic quality, the best crystal pictures can justifiably claim to be abstract works of art; but I have to accept that, at least for my lifetime, those who make them will continue to be regarded as geeks, unfit to be mentioned in the same breath as Jackson Pollock and Mark Rothko. Nevertheless, I dream that one day a lucky microscopist will spot an image on the slide that is unmistakably a Christmas robin, and will sell it to a publisher of greeting cards, thus taking the first step in a claim to be taken seriously. And then, who knows? One of us may find a poster-size enlargement of one of our pictures on the walls of Tate Modern.
by Theo Wyatt, UK . Comments to the author are welcomed.
Gallery of crystals taken between crossed polarising filters

Adipic acid 1
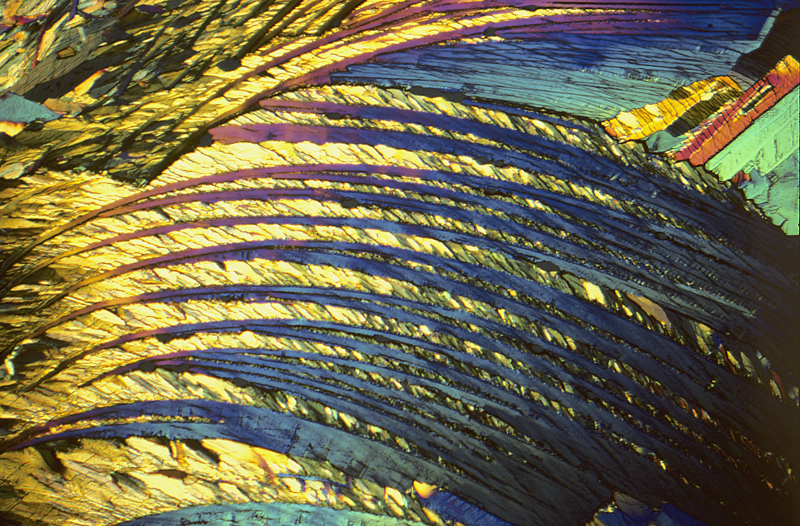
Adipic acid 2

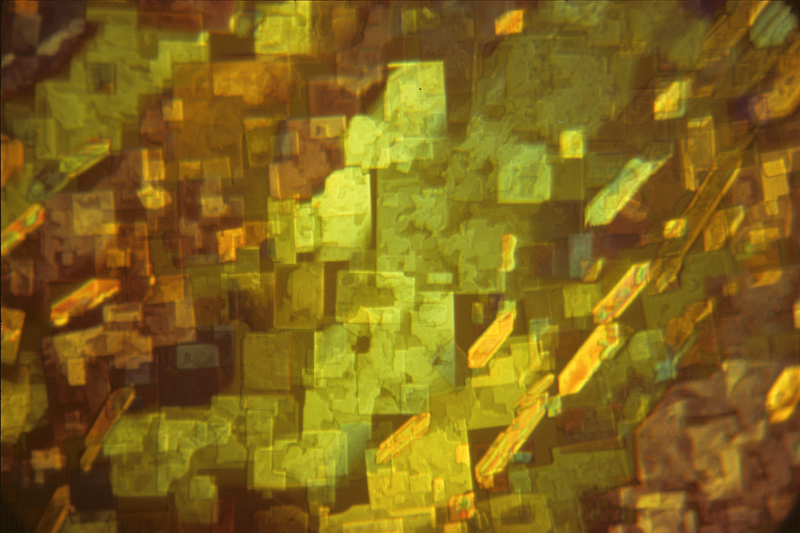
Benzoyl eugenol
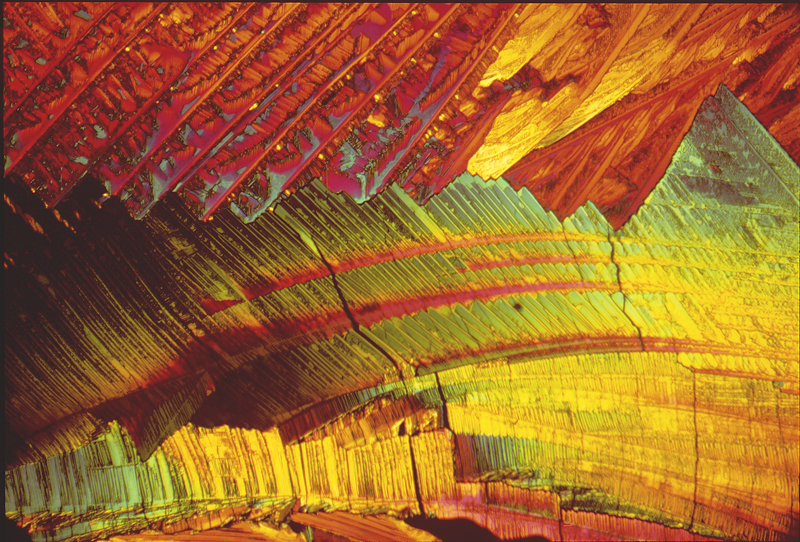
DMEM Stilbene
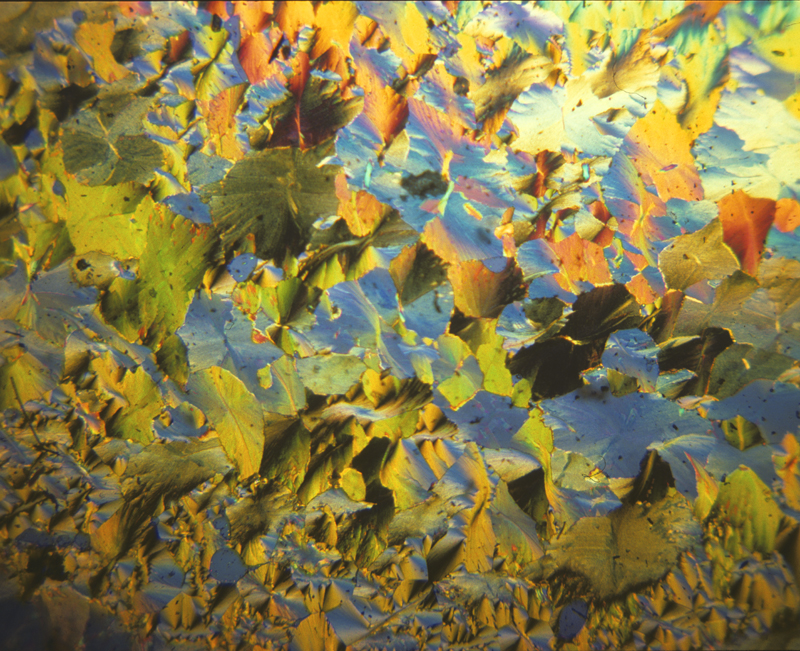
Diphenylamine

Ethyl para amino benzoate
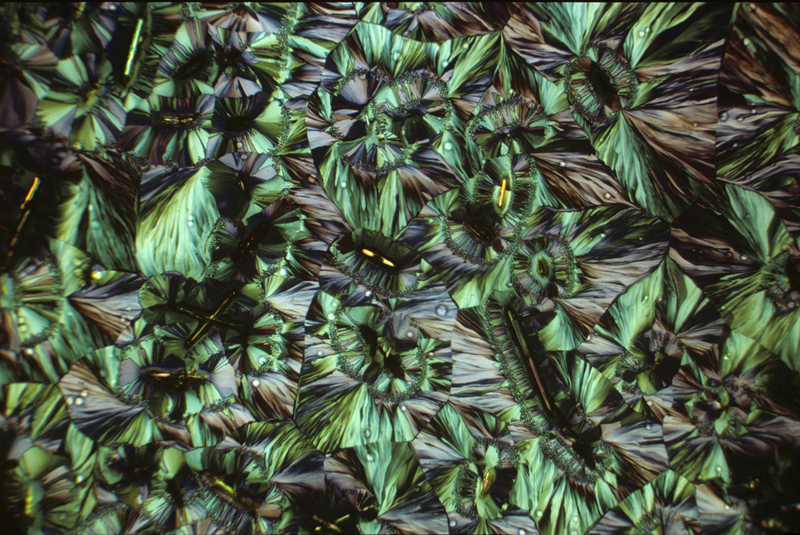
Hippuric acid 1

Hippuric acid 2

Hippuric acid 3
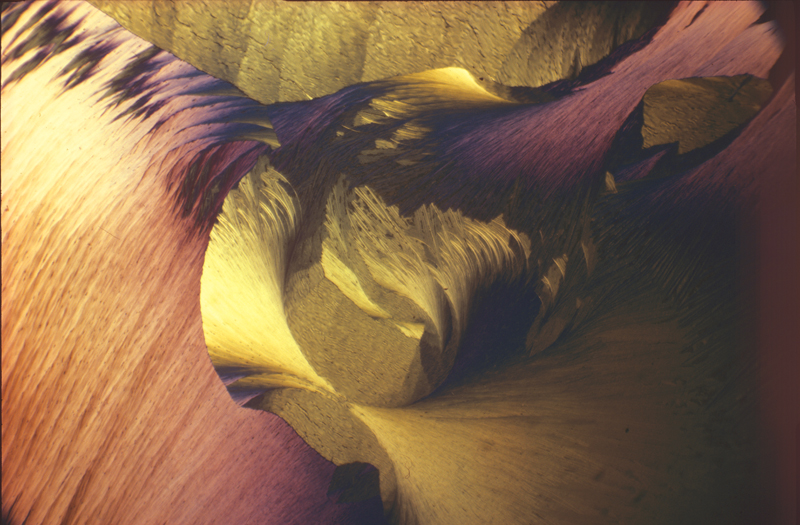
Lauric acid 1

Lauric acid 2

Lauric acid 3
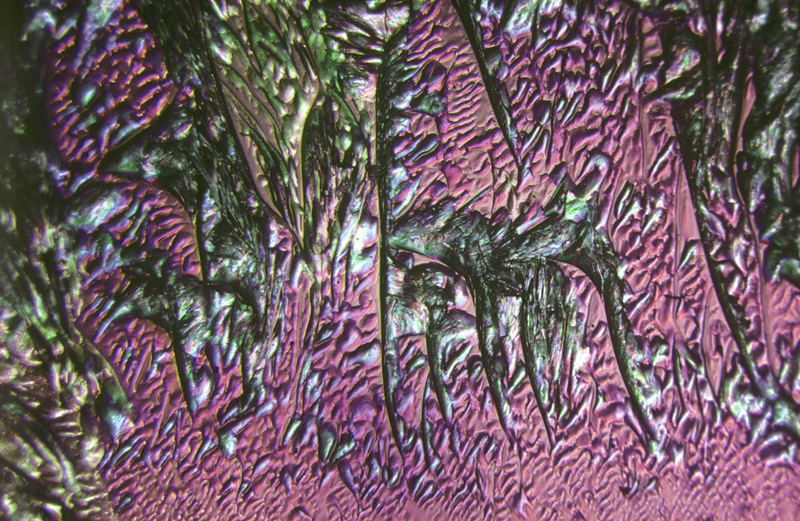
Lauric acid 4
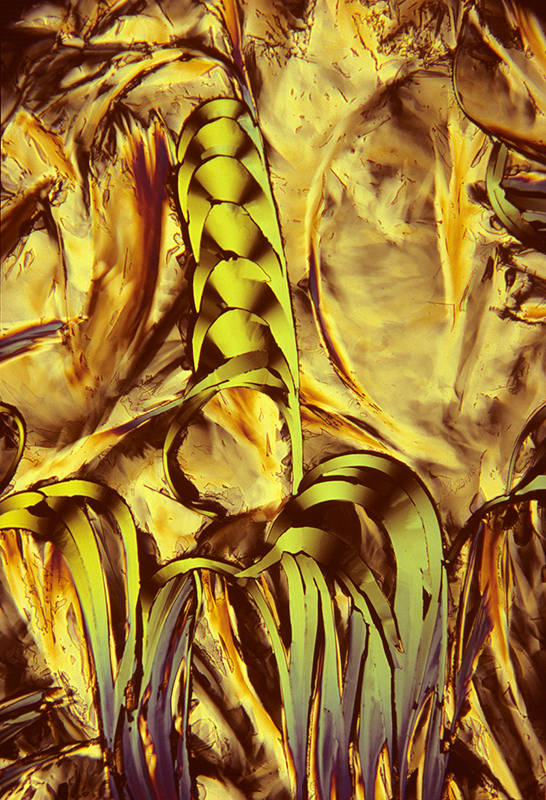
Lauric acid 5

Lauric acid 6
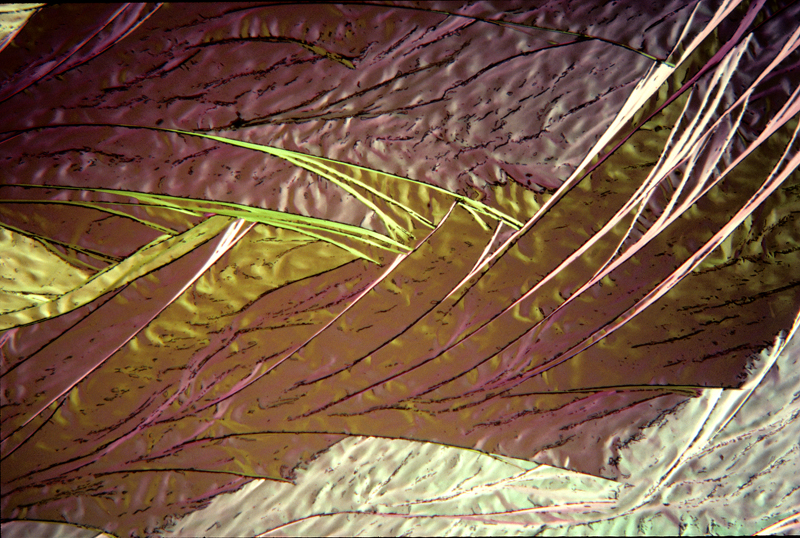
Lauric acid 7

Para hydroxyphenyl methyl ketone

P-chlorophenyl glyceryl ether 1
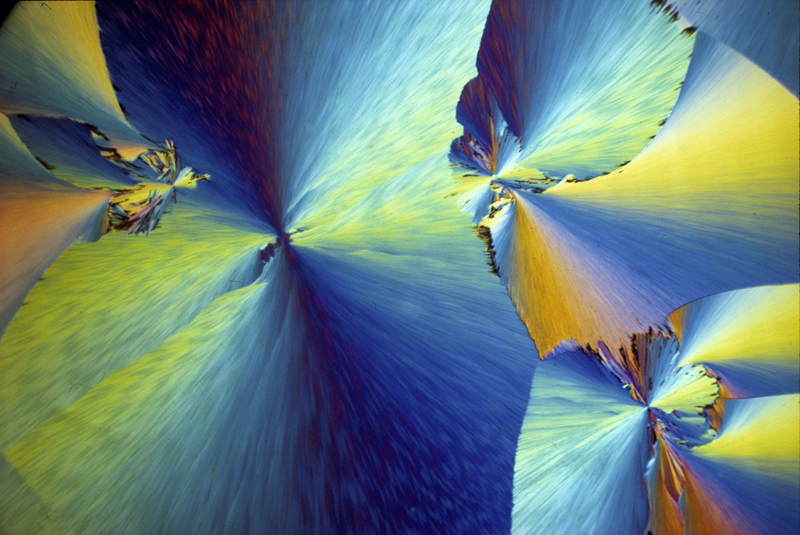
P-chlorophenyl glyceryl ether 2

Phthalic acid 1

Phthalic acid 2

Phthalic acid 592
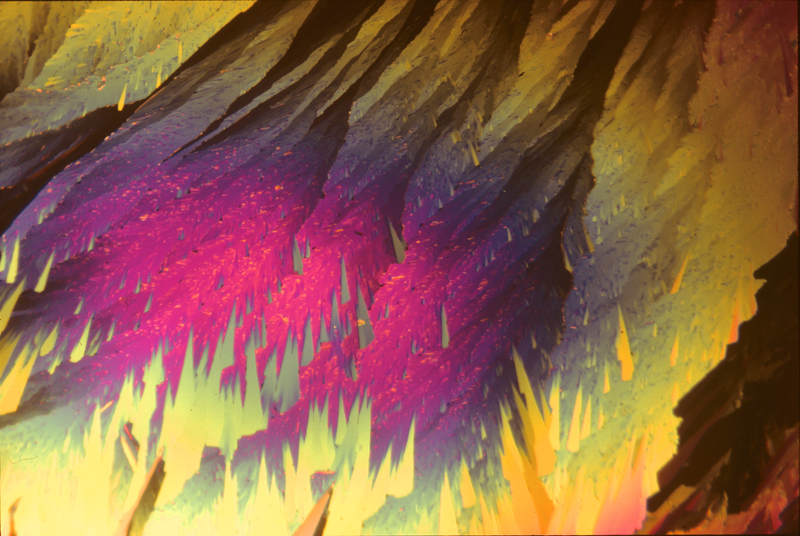
Piperonal
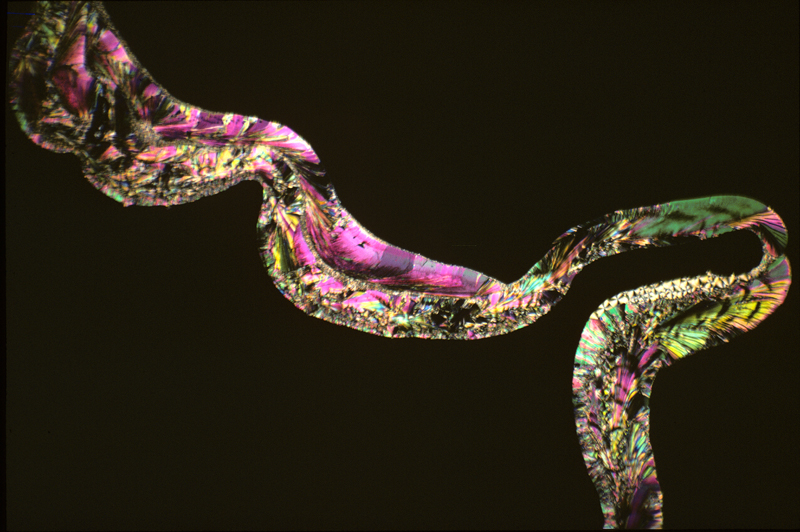
Salicin 1
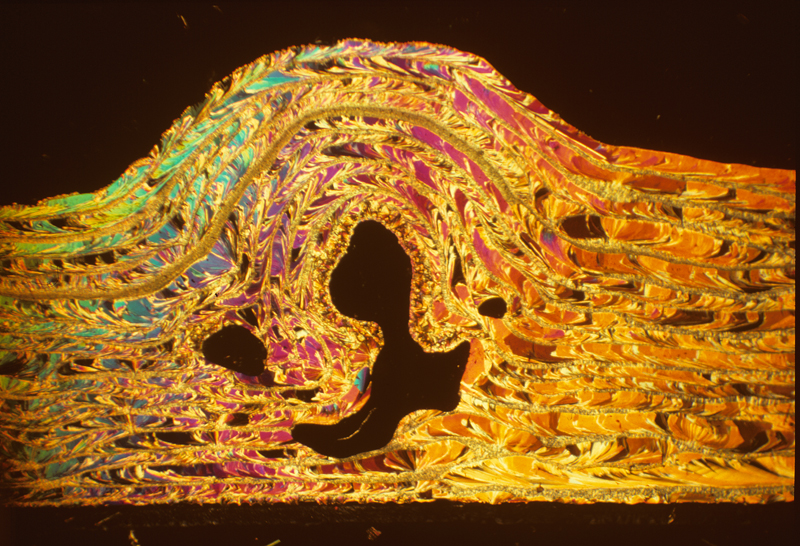
Salicin 2

Styryl methyl ketone
Published in the April 2016 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk.