Habitat for Lab Specimens and other uses for Common Household Items
Gordon Couger USA


Habitat for Lab Specimens and other uses for Common Household Items
Gordon Couger USA


When keeping live specimens in the lab we are faced with a wide range of conditions from aerobic to anaerobic and everything in between. Fortunately modern kitchen utensils and other things make it a lot easier to get small containers that are spill proof and can still be able to adjust the amount of air exchange without danger of spillages. Carol M. Stiff, Ph.D. on her Kitchen Culture Kits, Inc. site shows many ways of sterilizing baby food jars, food containers and using 3M water proof bandages as long term ventilation for containers that can be sterilized again and again in a pressure cooker or microwave oven.I used her ideas to make small habitats from inexpensive Gladware and Rubber Maid plastic food containers for pond life and terrarium for dry and swamp-like conditions that require very little attention, as the 3M band aids allow oxygen interchange with out excessive evaporation. To control the oxygen exchange rate you punch holes in the lids of the containers and cover the holes with the 3M Next Care waterproof Band-Aids. Using as many holes and band aids as needed to get the proper gas exchange. The 3M band aids reportedly can take repeated sterilizations and not lose their ability to keep bacteria from entering or escaping the container.
I have also use the 3M Band-Aids on Zip Lock bags to make temporary habitats that can be easily carried in the field. They are rather difficult to transport but for keeping something alive while on holiday a roll of Zip Lock bags and 3M Band-Aids can be bought at any grocery store.
These containers also make very handy collection containers and general lab ware. The lids are also handy for wet or dry trays for working under stereo microscopes. Back when I was doing photography some of them would have made nice trays for chemicals as well.
The larger flatter containers make excellent discretion trays if a mixture of paraffin, beeswax and Vaseline is melted and poured in the bottom. The mixture beeswax and Vaseline are added to keep the paraffin from crumbling when pins are stuck in it. This is paraffin wax similar to candle wax not what you Brits call paraffin.
Baby bottles make nice measuring jars.

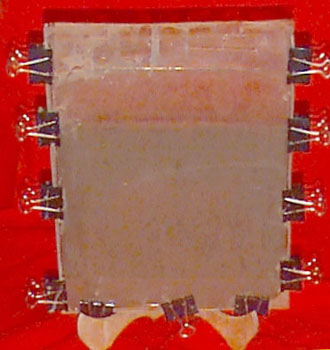
Going to the anaerobic extreme I was building a Winogradsky column which is a long tube filled with mud and topped with water that simulates a stagnant pond bottom using a 2 liter soda bottle, when I came across a paper by Peter John Charlton Ph.D.: 'The Winogradsky Plate, a Convenient and Efficient Method for the Enrichment of Anoxygenic Phototrophic Bacteria' that uses two pieces of glass and plastic sheets from an overhead transparency to make it possible to disassemble the apparatus called a Winogradsky Plate and get to the bacterial mat on the plastic sheet. Lacking the casting device for making electrophoresis gels, I built my plates by clamping the glass and plastic together separated by a piece of vinyl tubing and using strong paper clips to hold it together. This had the added advantage that the areas near the edge of the plate can be easily accessed with a hypodermic needle. So nutrients and other things can be easily added or bacteria can be removed with the syringe. The one illustrated used ¼ inch tubing. In the future I think I will try 3/8 inch tubing and use acrylic plastic instead of glass for the plates.Here are Dr. Charlton's comments on advantages of his plate over the conventional Winogradsky Column and some comments on his work following the publication of the paper.
"Shortly after publication of that brief methods paper, the focus of our research changed, and ironically we had little further use for Winogradsky-type enrichment techniques. I have spent the last four years studying photosynthetic bacteria from microbial mats in geothermal areas. These mats are gelatinous or rubbery, so certainly cannot be 'poured' between glass plates. I suppose thin horizontal layers could be cut, or traditional cylindrical Winogradsky columns set up, but it is not really possible to take large samples from a geothermal area, as such damage would take a long time to repair itself (and changes to the original population of organisms would be expected). In any case, most thermal areas in NZ are tourist attractions, and the Department of Conservation and the owner-operators do not take kindly to visible sample removal!Other members of the research group carried out lab-based studies of various physiological properties of purple non-sulfur bacteria. The senior researcher, my supervisor, Chris Harfoot, is retiring at the end of this year, and research activity has already come to an end, so I do not think there will be any opportunities to use the Winogradsky Plate system again.
I found, in fact, that the exploitation of quirks of physiology in rather 'brute force' selective growth media allowed me to isolate the organisms in which I was interested (mostly purple non-sulfur bacteria) more rapidly and reliably than Winogradsky systems would have. The latter technique still however has the advantage that it allows the potential discovery of completely unknown organisms. In this regard, Winogradsky columns are probably better than direct plating approaches, particularly due to the operation of ecological succession processes that may over time bring minor original community members to prominence.
I believe that some Scandinavian researchers intended to use the plate technique in the study of green sulfur bacteria (Chlorobium species), but I do not know whether anything came of it. Other than that, the JMM paper really sank without trace - which Jason McGrath and I fully expected! - With one amusing exception: the paper is cited on a web page that lists resource material for high school science teachers at http://www.woodrow.org/teachers/esi/1999/princeton/projects/microbe/resources.html
It would be nice if at least a few high schools around the US had Winogradsky Plates sitting in their windows!" - Peter John Charlton Ph.D.
As you can see my results have not been as spectacular as Dr. Charlton's sound but I am trying to digest vegetable oil in the dark so the growth is much slower. I am getting some growth particularly in areas enriched with acetate made by neutralizing vinegar with bicarbonate of soda in the zone where there is a little oxygen. It is the black line in the magnifying glass in the top right photo. You can also see some growth in the 1 liter soft drink bottle in the time between the picture taken on the red back ground and the picture in the top right corner of the page.In the future I will be writing more about soil microbiology but as I was putting this together it became obvious that there would be more general interest in labware than there is for microbiology from the Micscape readership. The online resources at the end of the paper have extensive links to resources on soil microbiology and Winogradsky methods for those of you that are interested.
Safety
I am not trying to scare you away from doing what you want but I do want to be sure that I impress on you that anything that can grow bacteria has risk. People all over the world deal with these risks every day but be sure you understand what the risks are, how to minimize the risk of an accident and what to do in case there is an accident. All our interests are different so we each must learn what risks we face and what risks are acceptable to us.I do not provide enough information about microbiology safety in this paper for you to proceed very far into microbiology. While most of my caution is about cuts and needles there are also some bacteria and fungi that can cause problems if inhaled. As long as you are working with pond dipping and even Winogradsky methods these are not great risks but don't leave old experiments laying around to dry out an mold. Get rid of them when you are done with them. Cooking them in the pressure cooker or burning them is probably the best way but for pond and soil experiments burying them is acceptable. Be careful flushing them down the loo as it can make an aerosol of the water in the loo.
When using food containers for experiments mark them well so there is no mistaking them for food and put them away so there is no chance for mistake. If you have children in the house it only makes good sense to keep the lab locked if you use anything that is dangerous. While you can educate your kids on safety you can't expect their friends to understand the danger of what is there or to heed your childrenís warnings.
I am comfortable working with most chemicals less toxic than mercuric chloride. My son moved away years ago and no children visit here that are not accompanied by my wife or I. The toxic chemicals are safely stored and I only work with toxic chemicals outdoors with protective gear. Everyone should decide what level of risk you are comfortable with and not exceed it.
There is risk in all things, even your aquarium can harbor amoebae that can cause fatal encephalitis, salmonella and other nasties. So don't work in the kitchen and be careful about washing your hands, containing spills and disposing of old experiments. If you are going to work with soil and needles, knives or glass make sure your tetanus shot is up to date and be careful of needle sticks. While extremely rare it is possible to get botulism injected into a muscle and the bacteria grow and produce symptoms of numbness of lips, weakness, flaccid paralysis and difficulty breathing and if untreated, death. This has literally a one in a million chance of happening. Much more likely are problems with abscesses and infections. While most of the bacteria are not very dangerous there are some in the soil that are deadly and if you stick yourself with a dirty needle and any symptoms develop attend to them immediately unless your tetanus shot isn't current. If you tetanus shot isnít current and you stick yourself with a dirty needle go get a tetanus shot. By the time symptoms develop it will almost certainly be too late to save your life.
I am very disappointed that there is not any text, web page or any other resource that I can direct you to that gives you a reference on safety that you can use and have some confidence about how safe your actions are on any subject. Safety has become such an issue that the people enforcing safety only look at the MSDS sheet without regard for what the process is that the product is being used for and want to eliminate all risk. On the Histology listserver one safety officer wanted the lab to find a substitute for formaldehyde. He wanted them to find a non-toxic fixative. Not only is it impossible to have a fixative that is non toxic, not using formaldehyde would probably render 80% of their work invalid. After they told him this he still wanted them to see if they could find a substitute.
The most important safety device is you. The way you think and consider your actions and their consequences before you act and how you react to an emergency can make all the difference in the world between a learning experience and something with serious consequences. I have a good friend who I began to call Lucky when he was driving a tractor, caught the plow on something and broke a 1-inch bolt and because he was looking back it knocked out all his front teeth. Had he been looking forward it would have hit him at the base of the skull and killed him. There is nothing that can be made safe for Lucky. He has blown up, burned, broken, cut off much of what is around him and himself. All the rules, safety shields and safety officers in the world can't make someone take the extra second to be careful.
In my opinion the most important part of safety is make it as difficult to have an accident as possible. Don't take the short cut, take the safe way every time. Don't store things that can cause problems were they can cause problems. Don't take unnecessary risks. Always do it the safe way and always think before you act. Think about safety as part of everything you do. Not just in the lab but the route you drive to work, how you prepare your meals and make it a way of life. Just like brushing your teeth. Itís not something you do when you need it. Safety is something you do all the time.
That doesnít mean not to take any risks at all. Just realize when you take them and pay real close attention to what you are doing and know what is dangerous and what is not. In todayís world it is very difficult to find out what is actually dangerous. When the one cow showing up with mad cow disease can wreck a nation's beef economy and the puffer fish that unless prepared exactly right can kill you are popular delicacy in the same country there is something very very wrong. Had the press not picked up Mad Cow disease it would only be a very small footnote because of the novel way it is transmitted. It affects about 300,000 cows, about 110 humans and a handful of animals at the zoo. I don't know of any disease that has this small a number of cases except for rare inherited disorders. I know people that raise organic food that are afraid of the sprays that conventional farming uses but won't test their grains and peanuts in particular for aflatoxins by seeing if it glows under ultraviolet light. I am concerned about the toxins in food as well but not to the point I buy organic. But I won't eat a peanut that I raise in my garden without checking under a lack light. Aflatoxins from molds are really serious toxins and it can be very bad on peanuts if the weather is just a little wrong. My point being is that many people will not bother to make a simple test for a well known toxin that can be a serious danger to them, but will make major changes in their lives on information that has less valid science behind it than the proof of the danger of the aflatoxin.
I used the example of agriculture because I know it well and it is in the news. But there are a great many fields that have the same problem. We can't get a true picture of the risks of many things and the current climate of safety officers and policy that believe that can make things safe with a capital S is making it much more difficult to establish safety policies that actually work. Every one of these bureaucrats needs to spend a week with Lucky.
I know people that experiment safely with a great number of things at home including nuclear energy. In the end you are responsible for your safety education and bear the ultimate responsibility for your safety. No matter how hard they try there is no way someone else can keep you from hurting yourself but to put you in a padded room in a straight jacket. So until you understand what is safe, if you err, make sure it is on the safe side.
Comments to Gordon Couger are welcomed.
Bibliography
1. The Winogradsky plate a convenient and efficient method for the enrichment of Anoxygenic phototrophic Bacteria.
Peter J. Charlton, Jason E. Mc Grath, Chris G. Harfoot.
A short communication to the Journal of Microbiological Methods, 30, 1997, 161-163Acknowledgement
Thank you to Peter J. Charlton Ph.D. for permission to use the quote in the article.Online resources
Winogradsky column: perpetual life in a tube hosted by the University of Edinburgh.
Using A Winogradsky Column to Analyze Microbial Communities by Frances Vandervoort, 1991 Woodrow Wilson Biology Institute.
A short biography of Sergei Winogradsky and a Winogradsky column by the American Society of Microbiology from their biofilms page.
NASA's page on building a Winogradsky column.
How to make a Winogradsky column from 2 liter soda bottles by The Ecosystems Center at the Woods Hole Marine Biological Labratory.
Another description of a Winogradsky column from Clemson University.
Instructions for a column for grades 7-12 by Ann Sowd Jackson High School, Massillon, Ohio.
Mud Biology from the Science Teacher magazine.
Kitchen Culture Kits Products is a commercial site on tissue culture for the amateur. It has a lot of information on how to grow cultures with minimal equipment such as using a microwave oven to sterilize media and sources for odd stuff that some may find useful. Kitchen Cluture is an amateur friendly source.
Please report any Web problems or
offer general comments to the
Micscape
Editor,
via the contact on current Micscape
Index.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK