|
Harris’ Hematoxylin: Fixing and Staining Protozoa by Richard L. Howey, Wyoming, USA |
For the amateur, achieving satisfactory fixation and staining of protozoans is often frustrating, difficult, and haphazard. There are a number of reasons for this. 1) Protozoa are extraordinarily diverse and are well-known for their propensity to play jokes on humans. 2) Some are remarkably hardy and can be subjected to rather strenuous chemical treatments and remain intact, whereas others have such a high degree of sensitivity that even slight alterations in the chemistry of their environment will induce lysis (when the organism’s membrane breaks open and its contents spill out.) 3) Many fixatives are extremely dangerous (osmium tetroxide, for example) and should be used only by professionals with special equipment, such as, a fume hood. So, at this point I need to introduce some cautions regarding Harris’ Hematoxylin before we proceed to any applications.
CAUTIONS Regarding Harris’ Hematoxylin
1) This fixative-stain contains mercuric oxide which is classified as extremely poisonous and corrosive.
2) Do not use metal implements with this reagent.
3) Use only disposable plastic or glass containers, pipets, etc. and put them in plastic bags before disposing of them.
4) Don’t drink this stuff! It tastes terrible and will give you an awful hangover. Seriously, while there is relatively little mercuric oxide in this solution, it is nonetheless sufficiently dangerous to take reasonable precautions. Wear rubber gloves when handling this reagent. The toxins can be absorbed through the skin.
5) Buy the solution already made up. Do NOT try to prepare it yourself!
Why Use Harris’ Hematoxylin?
1) It’s relatively inexpensive, since a little goes a long way.
2) Although it contains relatively little mercuric oxide, it is nonetheless a very effective, quick-acting fixative and in relation to many other fixatives produces rather little distortion in many organisms. Sometimes one can even get good results with highly contractile organisms, such as, Vorticella.
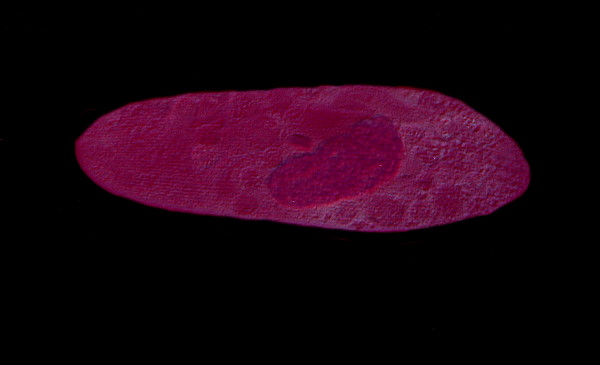
Consider 3 images of Paramecium below.

Here the nucleus of the Paramecium is very distinct and as you can see there is relatively little distortion in the membrane.
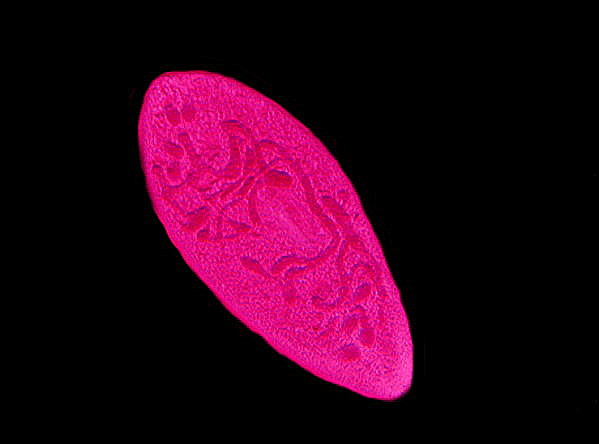
Paramecium again, but this time a specimen in which the nucleus was dividing.
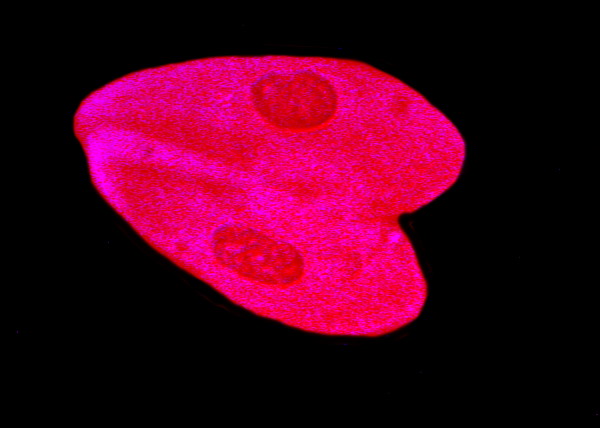
Paramecium, yet again, and in this case we have 2 organisms conjugating. These images were taken from slides which I made about 10 years ago. They were made using a drop of rich Paramecium culture over which a cover glass was placed. I then introduced a drop of Harris’ Hematoxylin at the edge of the cover glass and allowed capillary action to gradually pull the stain across the flattened drop. If you introduce the stain at the left side of the cover glass, the organisms at that edge will either receive a full dose or quickly flee to the middle or right edge of the drop. As capillary action pulls the stain across, a gradient is produced with slight dilutions of the stain so that you get delayed responses from some of the organisms. With some protozoa this procedure may not produce rapid enough fixation to avoid distortion or lysis. In such instances, try putting a small drop of the culture in the center of the slide and then adding a drop of Harris’ Hematoxylin and quickly mixing with a flat toothpick. Add a cover glass. Put the toothpick in a plastic baggie along with any pipets and discard slides that have come in contact with the stain.
3) Certain cytological elements are also effectively stained by this reagent. One should first acquaint oneself as fully as possible with the structure of a given organism by observing it live. This is useful for determining artifacts which may show up in the organisms–objects such as Zuni pots, Greek kraters, or shards of Roman glass which could have enormous archaeological value. Oops, wrong kind of artifacts. The kinds of artifacts that microscopists have to watch out for are those that result from highly complex chemical reactions between the reagents and the organic (and, in some cases, inorganic) constituents of the organism. Such reactions can produce deposits which look like structural elements of the critter, but are essentially complex precipitates. A famous parallel sort of case was Ernst Haeckel’s notorious Urschleim or primordial slime. After examining jar after jar of preserved marine specimens which at the bottom of the containers held this amorphous, gelatinous, undifferentiated goo, he came to the conclusion that this was the “essential stuff” from which life had evolved. As it turned out, it was a precipitate formed as a consequence of the salt water in the specimens reacting with the preservative.
Artifacts are more common than one might think and so, if you are doing serious work on an organism or group of organisms, it is highly desirable, if possible, to use a series of contrast techniques on living organisms–brightfield, phase contrast, darkfield, oblique illumination, Rheinberg, Nomarski Differential Interference Contrast, and Fluorescence. In some cases even polarization may give you helpful information. Few amateurs will have all of these available, but the more such methods of observation you can employ, the better. A second approach is to use several different killing agents and fixatives and then compare the results and look carefully for anomalous structures that might turn out to be artifacts.
As I mentioned earlier, some of the slides which I made are about 10 years old. They have a cover glass, but no mountant. They must be stored flat and handled carefully in order to avoid having the cover glasses fall off. Although some evaporation has taken place, my preparations have never completely dried. I suspect that my solution–which I bought commercially–has a bit of glycerine added to it. This is an advantage not only in preventing the slide from completely drying out, but also in helping to prevent the deposition of mercuric oxide crystals on the slide. The traditional formula for Harris’ Hematoxylin does not include glycerine, but individual researchers and biological and chemical supply houses often modify formulae and this is quite common for biological stains. In the 19th Century and the first half of the 20th, biological scientists were constantly trying out new formulae and techniques. That is less true today when researchers tend to rely on technology and a “catalog” of more or less “standard” techniques and reagents. Now, if such experimentation takes place, it is usually done by graduate students or laboratory technicians.
Perhaps the three best know hematoxylin stains are Delafield’s, Harris’, and Ehrlich’s, but there are at least a hundred formulae for other types and variants. So, I am not at all surprised that I ended up with a version that contains a bit of glycerine. If you want to make “permanent” mounts out of these preparations, then you will probably have to go through the procedures for removing the excess mercuric oxide from the slide and then go through the rather tedious process of dehydration to mount the specimens in Canada Balsam or a synthetic resin. Frankly, I don’t want to be bothered. If I want slides that last more than 10 years, I’ll simply make sure the area around the edges of the cover glass are thoroughly dry and then apply several coats of varnish to seal the preparation.
The lazy man’s solution–well, yes, I admit it. With the advent of digital photography, there is less need for certain kinds of preparations to be kept as “permanent” slides.
There are some protozoa that are quite delicate and cannot endure the rather harsh treatment of being subjected to powerful fixatives, such as those that contain mercuric oxide. For some time, I have been sporadically exploring the use of copper acetate as a killing agent. It produces a very delicate fixation and must be followed up with a hardening agent. I hope to be able to bore you with an essay on this subject in the relatively near future.
All comments to the author Richard Howey are welcomed.
Editor's note added Aug. 14th on behalf of the author: The article's mention of mercuric chloride has been corrected to mercuric oxide, the correct chemical in Harris' hematoxylin. Thank you to Gus Wanner for pointing this out to the author.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .