Preparation of Crystals for Observation
Robert Pavlis, Girard, Kansas USA
Crystals of many organic and inorganic compounds are very interesting and beautiful things to study using polarising microscopes. (We are using the term "organic" here in the way that chemists do—covalent compounds of carbon.)
Although there are many compounds that are hazardous, there are huge numbers of compounds with low toxicity that are at least as interesting to examine as the dangerous ones.
Commercial preparations of most substances are almost always highly unsuitable for microscopic examination because crystal quality is seldom of interest for manufactured chemicals. There are several techniques that can be used to make crystals suitable for microscope examination that require virtually no special equipment.
Crystallisation from solvents in Test Tubes.
Organic chemists generally purify the solid compounds that they produce by a process called recrystallisation. Practising organic chemists perform this process so often that it is almost a ritual for them. The standard technique calls for dissolving the impure compound in a suitably sized flask of boiling solvent, filtering off any solid impurities, and then allowing the filtered solution to cool as slowly as possible. Because most substances are much more soluble in hot solvents than cold ones, the material crystallises from the solution as the solution cools. Typically after several hours the organic chemist then separates the solid from the solution using a special filter called a Buchner filter and then the crystals are dried.
A variation of this technique can produce extraordinarily good crystals for microscopic observation. The microscopist is generally interested only in producing a solution with well formed crystals suspended in it—not dry crystals. Microscopists typically prefer smaller crystals than research chemists who are trying to produce ultra pure materials.
The first task whether purifying compounds or producing suspensions of crystals for microscopy is to find a suitable solvent. Unless one has suitable laboratory facilities available it is best to restrict solvents to either water or small alcohols such as ethanol, propanol, or butanol. Organic chemists probably use water and ethanol for at least three quarters of their recrystallisation procedures. (An experienced person might also perhaps consider using toluene, xylene, or materials like heptane.) Water is always best for microscopy! It is not corrosive, toxic, nor does it ignite and burn. Many oxygen and nitrogen compounds crystallise very well from it. However, it is a waste of time to attempt to crystallise other organic materials from it—they simply are not soluble in at any temperature.
To do this one needs only test tubes, droppers, and some sort of heating device. One of the best heating devices is a simple butane torch. The procedure is very simple. Put a few crystals of the compound in the small clean test tube. Add enough solvent (remember water is the best first choice) to cover it about one centimetre. Heat to boiling. This must be done carefully, because solutions tend to super heat, and the material can come flying out of the test tube. Do not aim a loaded test tube at anyone! If the crystals dissolve, remove them from the flame and cool quickly. Generally the crystals are smaller when the material is cooled rapidly, and this is really usually very desirable, because slow cooling is apt to produce huge crystals that are too large for microscopy. If the compound has the right solubility characteristics the test tube will soon be filled with crystals. When the test tube has cooled to room temperature, a few crystals can be drawn off with a dropper and this preparation can placed on a side, a cover slip can be added, and the result can be placed on the microscope stage. Note that this does NOT produce a permanent preparation!
Should water fail to produce crystals one can try other solvents, ethanol is, as stated above, often a very good choice. One can also mix ethanol and water should the material be too soluble in ethanol and not soluble enough in water. Ethanol, however, can ignite fairly easily!
When the material being examined contains insoluble impurities, it may be necessary to filter the solution. A convenient way to do this without any special equipment is to take a paper towel and cut a circular disk from it about three or four cm in diameter. This can be folded in quarters and then opened to make a filter which can be placed on top of a test tube. It is best to avoid using conical filters for small volumes, because most of them are much too large.
Heptane and similar solvents often work very well, but they are a fire hazard. Toluene and xylene are much more toxic, and are at least as bad as fire hazards. (Ether is almost always a bad choice, it boils just above room temperature, and it ignites on heating only to 190 degrees C!)
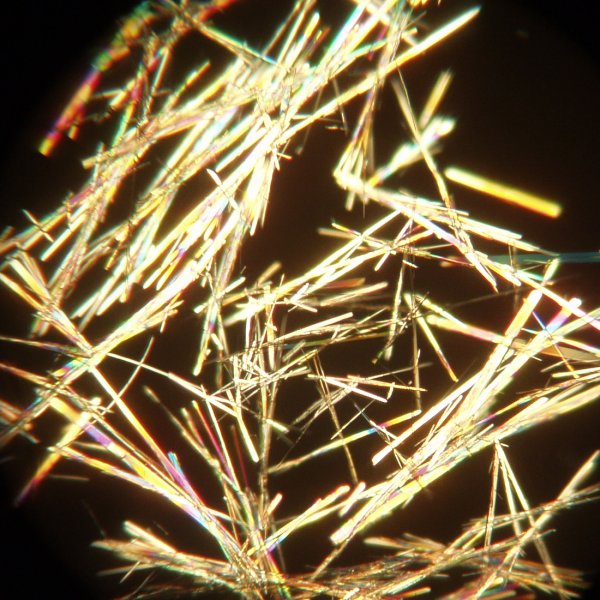
The image below shows the plant hormone mimic 1-napthylacetic acid crystallised in this manner using a mixture of ethanol and water and using a 4x objective. This compound has a tendency to separate an oil when the mixture is cooled too rapidly.

Molecules that are not particularly symmetrical and that are fairly complex often crystallise from solvents rather slowly.

Crystallisation from solvents on Microscope Slides.
For highly soluble compounds crystals can often be produced by taking a saturated solution of the material (or near saturated solution) and placing that on a microscope slide and allowing the solvent to evaporate.
Crystals made in this fashion are often not very satisfactory, especially when the material is highly soluble in the solvent.
There are some conditions, however, where this technique works very very well. Many compounds are highly soluble in ethanol, but much less soluble in water. Such compounds very often dissolve reasonably well in a 50-50 mixture of water and alcohol. When a compound dissolved in such a solution is placed on a microscope slide, the ethanol tends to evaporate first, causing the compound to crystallise. (This technique very often also works using high boiling point alkanes mixed with high vapour pressure solvents like dichloromethane, chloroform, or benzene. However, dichloromethane, chloroform, and benzene are quite toxic.) When this technique is used, it is best to place a cover slip over the material as soon as a good crop of crystals forms, so that the crystals can be observed suspended in the less volatile solvent.
Crystallisation from solvents on Heated Slides.
Instead of placing solutions of compounds on a slide and obtaining crystals by allowing the solvent to evaporate, one can also place a tiny quantity of the material on a slide, add a drop of solvent, and then heat the mixture with a butane torch or other heating device. It is VERY easy to ignite solvents like ethanol and toluene this way! Anyone trying this should choose the work area carefully!
One of the best ways to observe the polymorphs of sulphur is to crystallise it this way using nitrobenzene. Nitrobenzene, however, is extremely poisonous, and very easily ignited.
Although this technique is often recommended, crystallisation in a test tube generally provides better crystals and is less likely to result in a fire for non-aqueous solvent systems.
Crystallisation from melts on heated slides
One can place a few crystals of the material being studied on a slide, add a a cover slip and then heat the material with a butane torch or electric heater to melt the material. On cooling a solid mass of crystals will form between the slide and cover slip. Crystals made this way are very different from other techniques—they typically grow into one another forming a complex mat.
This is certainly the best way to observe "liquid crystal" materials. For most other materials it is often very unsatisfactory.
Sublimation
Some compounds have sufficient vapour pressure at temperatures far below their decomposition temperatures to enable them to be sublimed. Sublimed crystals are often extraordinarily good for microscopic examination.
A good way to do this is as follows. Take some 1mm diameter wire (#18) and wrap two pieces of it around the ends of a standard 25x75mm slide. Place a small amount of the compound to be sublimed in the centre of the slide. Place a second slide on top of the first. (The wires will hold the second slide about 1mm above the first—ideal for this) Being careful not to burn your fingers, heat the centre of the slide with a burner such as a butane torch. If you heat too strongly you may break the slide. If the compound have sufficient vapour pressure, crystals will form on the upper slide. You can take a flat piece of metal and lie it in contact with the upper slide to cool if you find that you cannot get the material to condense.
Naphthalene, benzoic acid and phthalic anhydride are two common materials that sublime this way. Mercuric iodide sublimes this way too, but it is a rather toxic substance, and requires a properly equipped fume cupboard for safety. Mercuric iodide is interesting because freshly sublimed material contains two different polymorphs, a red one and a yellow one. The yellow crystals slowly change to red.
Insoluble Inorganic Salts
Probably the best way to produce small crystals of insoluble inorganic salts is to prepare them on the surface of a microscope slide by a meta thesis reaction. The technique is quite simple.
Suppose one wanted to prepare calcium oxalate crystals, it is highly insoluble. One can prepare a small amount of a strong solution of calcium chloride (very soluble) and a second solution of sodium oxalate (soluble). A drop of each of these solutions can be placed side by side on a slide. A needle can be passed between the two drops to create a channel for them to mix. Crystals will form as the materials very slowly mix. (Simply mixing the materials often results in ultra small crystals too small to study.)
This technique can be employed for micro qualitative analysis of many materials.
An Example: Aspirin
Aspirin is a common name for the acetate ester of o-hydroxybenzoic acid. It is sparingly soluble in water, and much more soluble in ethanol. Commercial aspirin tablets contain aspirin, but they are highly unsuitable for microscopic examination without recrystallising them in some manner. These tablets also contain insoluble binders that must be removed by filtration. However, the only required equipment is two test tubes.
Crush an aspirin tablet into a fine powder. Put the powder and about 3 millilitres of 95% ethanol in a small test tube. Short ones with fairly large diameters are preferable.
Next cut a circle of paper from a paper towel, fold it into a cone and fit it to the top of a second test tube. Make the final cone so that its diameter is slightly greater than the test tube.
Heat the ethanol solution Carefully heat to boiling., a butane torch works well, but one can also use an ordinary stove. Be careful not to set it on fire!
Pour the solution through the paper towel filter into the second test tube. The resulting solution should be reasonably clear, though it may be slightly cloudy, this does not matter as long as the turbidity is slight.
If one place the ethanol solution on a slide it will produce a flat mesh of crystals. They are interesting to observe, but very poor material for studying.
Excellent crystals can be obtained by diluting the filtered ethanol solution with two volumes of water and placing a drop of the resulting mix on a slide. The ethanol will evaporate leaving mostly water, and as the ethanol concentration falls crystals will form. These crystals are generally very well formed, and can be used for crystallographic examination.
They also not only are suitable for crystallographic observation, they are very beautiful! The images below are of aspirin crystals produced in this manner. At low power, using a 4x objective, the material shows needle like crystals that are much shorter than those from 1-naphthylacetic acid.

Using the 10x objective the crystals show more detail.
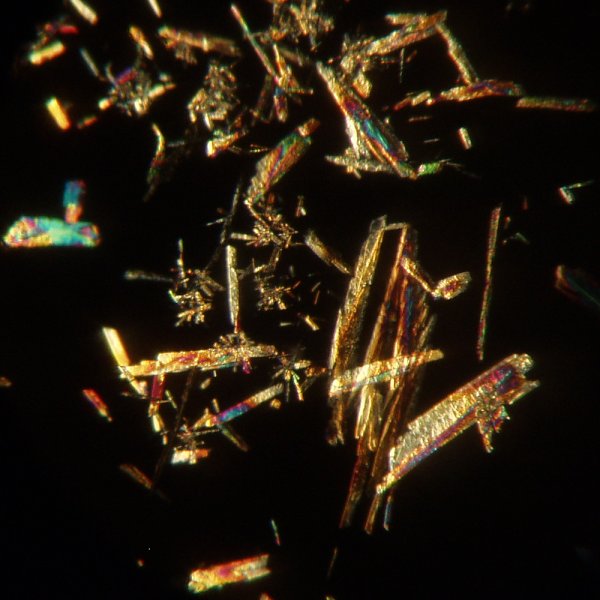
Another view at 10x:

The views using a 25x objective are very interesting.
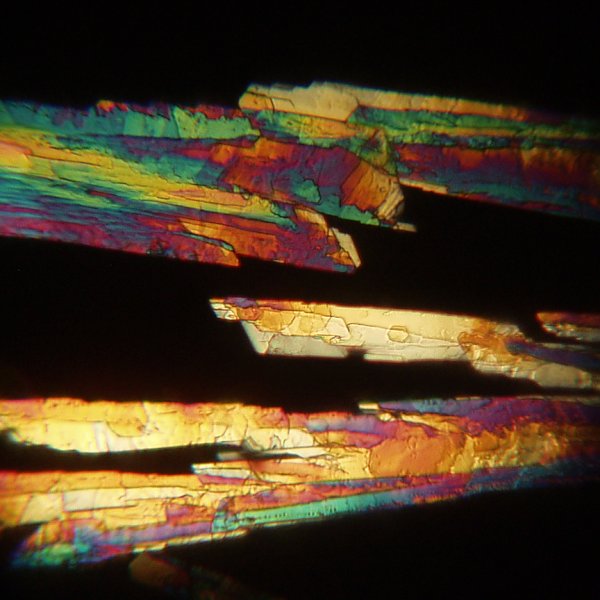
The image below shows a different field of crystals.

When the solution was very concentrated, a spatula was run through the super saturated solution. This caused a mass of crystals to separate where it touched the solution.
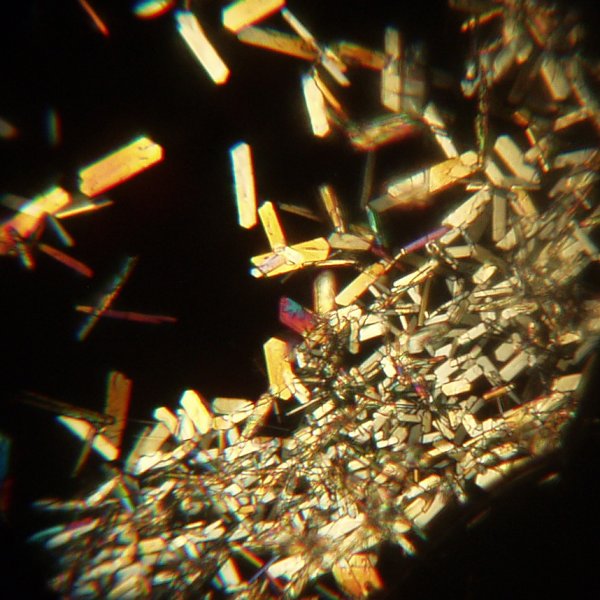
Safety Considerations
Heating water and most other materials in test tubes can result in superheating the material. The material can suddenly violently "burst" into a boil. The chances of having this happen can be reduced by using larger test tubes and be shaking the solution while it is being heated. This problem is much more severe when materials are heated rapidly. One can suffer burns by being hit by solutions violently expelled from test tubes. As stated above, never point a test tube that is being heated at anyone.
Be careful to avoid igniting flammable solvents such as alcohol. Use them only in areas where there is no danger of starting major fires.
Avoid needless use of highly toxic materials.
All comments to the author Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK