The
inner epidermis of the onion bulb cataphylls
(the
onion skin)
Easy
and not so easy methods to work with
Walter Dioni
- Cancún, México
5) fixing with alcohols
Continues from Part 4 – fixing with acetic acid
Alcohol
as a fixative
The adventure with the “ceviche” (see previous
articles) made me think of other ways to preserve food that may have been an
incentive for the experimentation of the first histologists.
I discarded sodium
chloride, the “salt” (dry salt, and brine), because their products are undoubtedly
preserved, as salt dehydrates (even the bacteria, making it inoperative, thus
its utility), but also hardens, and usually deforms. (Do you remember the dry salty pieces of cod?). However, I picked up some documents which show that salt was
used earlier as a stabilizer, mixed with other products, to preserve animals
and even humans for anatomical research.
But I remembered (Oh! Yes!) the beautiful cherries my
aunt packed every year in sugar and alcohol. And I immediately visualized the
rows of bottles in the collections of museums, many of which are full of
alcohol to preserve their content, which thus lasts for dozens of years.
I think that the following short historical resume about
the relationship of alcohol with human beings is clear and sufficient (more
data in Wikipedia):
Ethanol has been used by humans since prehistory as
the intoxicating ingredient in alcoholic beverages. Dried residues on 9000-year-old
pottery found in northern China imply the use of alcoholic beverages even among
Neolithic peoples. Its isolation as a relatively pure compound was first
achieved by Islamic alchemists who developed the art of distillation during the
Abbasid caliphate, the most notable of whom was Al-Razi. The writings
attributed to Jabir Ibn Hayyan (Geber) (721-815) mention the flammable vapors
of boiled wine. Al-Kindi (801-873) unambiguously described the distillation of
wine. Distillation of ethanol from water yields a product that is at most 96%
ethanol, because ethanol forms an azeotrope with water. Absolute ethanol was
first obtained in 1796 by Johann Tobias Lowitz, by filtering distilled ethanol
through charcoal.
http://e85.whipnet.net/ethanol.history/
I have records of its use in histology from the very
beginnings. Absolute (100%), 96%, 90%, 70%, and “1/3 alcohol” were used
for different purposes. Apparently a consensus was rapidly reached that to fix, the
best concentration was Absolute
Alcohol, even if this is very difficult to
maintain “absolute”. Absolute alcohol was selected as the best plant material
fixative in the 1884 edition of the famous Strasburger's "Botanische
Prakticum".
Their more useful role was, and is until now, as a
component of most of the most credited fixative formulas, in a variety of
concentrations. In short, most writers say that its merit is to surprise the
cytoplasm, taking out its water suddenly without giving opportunity for severe
changes in their structure.
To be coherent with the design of this series I did a rapid test of the alcohols within my reach, as fixatives for the onion skin,
using this to highlight the specific modalities for its use.
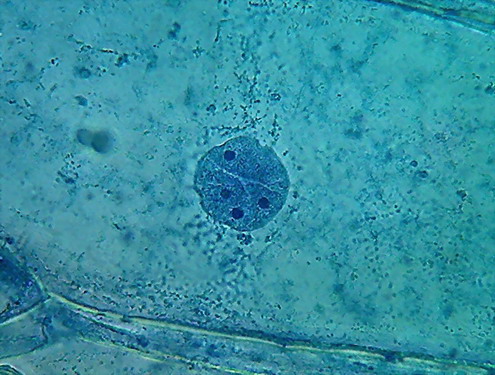
96% ALCOHOL
I am working with a plant tissue whose cells are bound
by a quite rigid wall of cellulose. If alcohol pumps out water from the cells cytoplasm
its volume must reduce.
So I did a first quick test to verify if, as one might
think, strong alcohol produces plasmolysis. I fixed in pure 96% alcohol for a
period of 15 minutes, and examined a sample in the same alcohol. I could detect
no effect greater than that produced by the reagents I used before!, so I made
a formal test slide, using alcohol 96% and the Blue 1, as I have until now.
I follow this protocol:
Fixation: alcohol 96,
15 minutes; alcohol 70, 5 min.; alcohol 35, 5 min.; distilled water, 5 minutes; Blue 1 (according to the result of a
previous experience) 1.5 minutes. Washing
until no more clouds of dye were released, rinse in another water bath and mounting.

Fig 1 – the reagent series
Hydration: To avoid too much tension to the nucleus and the
cytoplasm if I go directly from the alcohol to the aqueous dye, I set 3
hydration steps. Descending the concentration from 96%, through 70%, to 35% and
finally to water.
Normally, in professional histology, and especially in
the treatment of sections made with a microtome from animal material, a much
more delicate hydration series is performed when a high alcoholic fixative is
used, varying the concentration by only 10 or at most a 20 per cent in each
step. Not doing so produces artifacts by creating for example false cracks
between naturally contiguous structures.
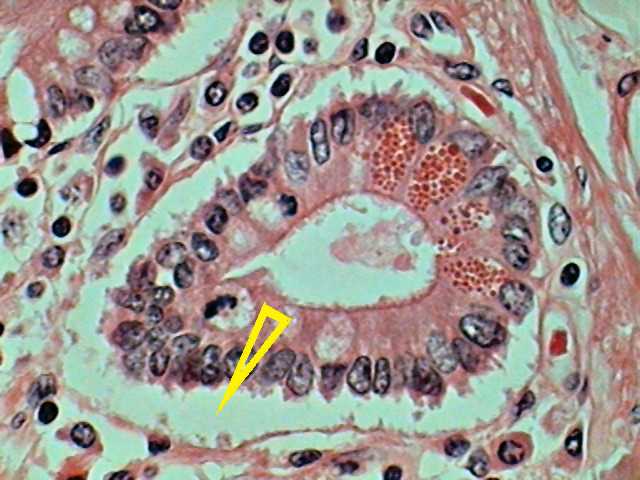
Fig. 2 – Duodenum, section of a Paneth gland, Harris
Haematoxylin – Eosin. The arrow points to a crack artifact due to inadequate
dehydration of the histological piece. 100xOI objective. DC3 camera. From a
commercial slide.
However, still wishing to be careful in my
preparations, with only 3 steps, I did not find severe deformations
attributable to fixing.
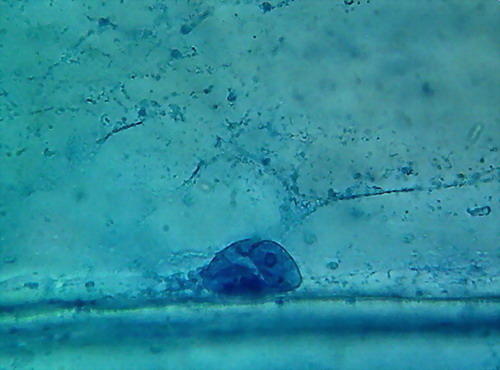
A disappointing detail is that preparations, while
they show clean spacious fields, are also riddled with bubbles.
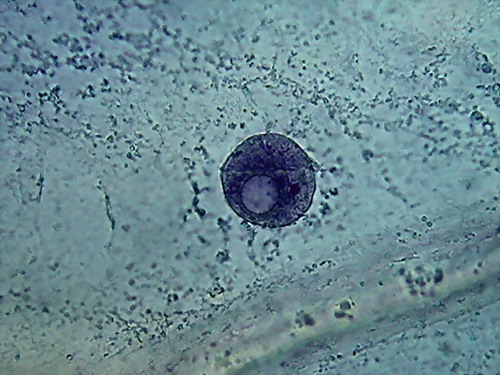
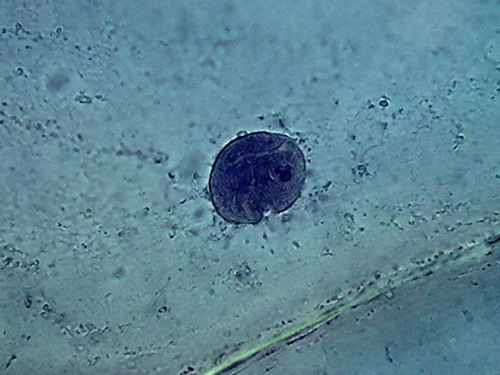
Fig 3 -
4x objective, 96% alcohol
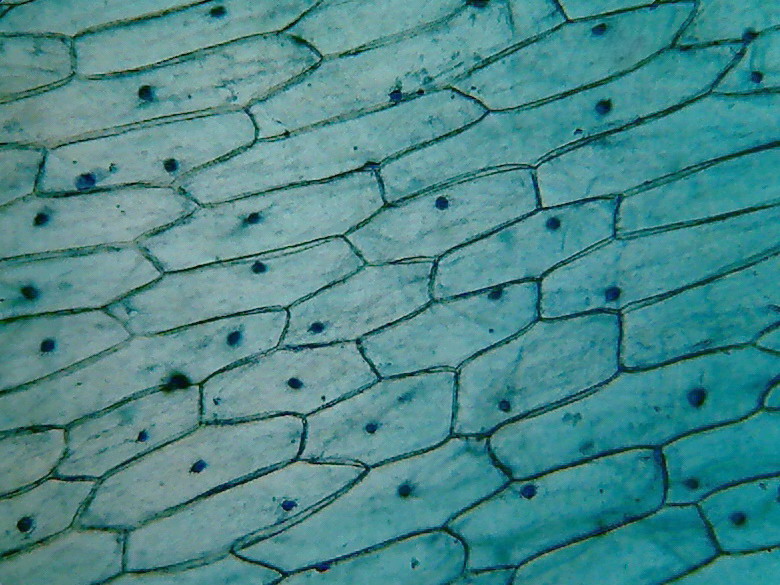
Fig 4 - With the 10x objective, however,
you can find good fields to work with
|
|
|
|
|
|
|
|
|
Fig 5 - 100xOI obj. – 6 pictures (Logitech
9000) reduced to ¼ its size. Comments in the text
In almost all preparations made with the methods I
used before (with the notable exception of the Iodine tincture – see first
article) cytoplasm appears as a transparent area, perhaps with a weak more
refractive layer of cytosol attached to the cell wall and some scattered
granules or irregular granular spots and bands.
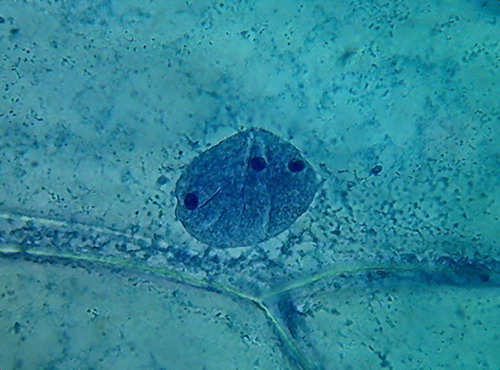
In this case it's remarkable the good appearance of the
cytoplasm, in which a layer of fine granules along the cell walls are easily
identified (pictures 2 and 3) with accumulations in the corners (see picture 5 of
the upper series), and also coarsely reticulate granular areas close to the
walls (top or bottom) of the cell, as well as the suggestion of bands of
granules (without visible bounds), around the nucleus, and which extends radially
from the same (fig 4).
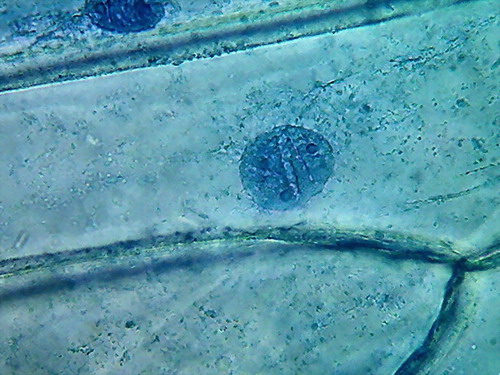
Indeed, 96% alcohol has made the cytoplasm far more
visible, than with previously used fixatives. Cytologists say that the small
granules are mitochondria, and vesicles, and other granular materials included in the cytosol.
I know that live cytoplasm is structured. The two dark
background pictures I posted in the first part show this. My hope is to show,
with techniques that anyone can repeat, the delicate aspect of this living
structure. Alcohol seems to be a step towards this end.
Nucleoli are very visible here, as small dark areas (much
smaller than with the other fixatives).
The most impressive detail is the appearance of the “grooves”, that with the other fixatives were seen as more or less deep folds, visible, but not ostentatious, which here are seen as neat, short, broad and deep incisions spanning the nuclear disk. I interpret this difference as
due to the strong dehydration of the nuclei.
70%
ALCOHOL
70% Alcohol is widely used in macroscopy,
to preserve the sampled materials for further study. 10% Glycerine is generally
added for to protect the pieces against alcohol evaporation.
Strangely it is not so recommended for histology,
where histologists use the much weaker alcohol at 30% approx. As you
can see below I believe alcohol 70, applied to the skin of onion is a simple
but helpful Fixer.

Fig 6 – The reagent series
Fixing: 70% Alcohol: 15 minutos, 35 % alcohol, 5 min., water, 5 min, new dye solution, 2 min., washing,
rinse.
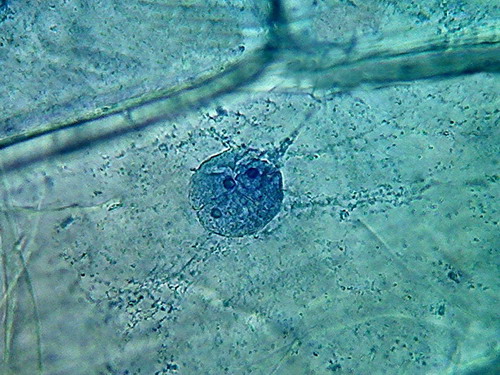
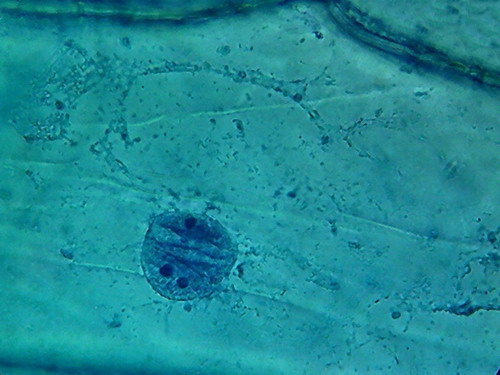
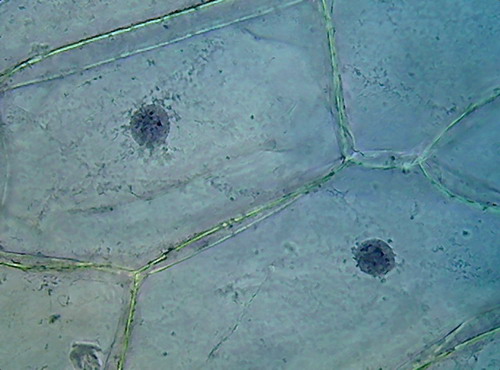
There is a big difference between both pieces (96 and
70%). 70% Alcohol provided a more clean piece, with the nuclei very well fixed
(according to the experience gathered so far), very evident nucleoli of normal
size and strong staining, and a cytoplasm with a well fixed lattice (see photos
5 and 6 of the following series), and good relations with the nucleus, although
96% alcohol shows a more natural distribution of the mitochondria.
|
|
|
|
|
|
|
|
|
Fig 7 – 6 pictures of nuclei and
cytoplasm fixed with 70% alcohol
35% ALCOHOL
French
histologist Louis-Antoine Ranvier (1835-1922), proposed and promoted the use of
the "1/3 alcohol" known at his time just as "Ranvier
alcohol", which he produced by adding 1/3 of 90% alcohol to 2/3 of distilled water.
Today
it's easier (and especially for an amateur) to use a 50% dilution of the common 70%
alcohol, which is why I'm trying alcohol at 35%.
Bolles
Lee (and the ten editions of his Manual that are published at the Internet
Archive), is unavoidable. On any subject it has and offered an opinion. Many
times it is not very understandable at first reading, because the described
concepts have widely varied.
For
example, he classified reagents as fixatives
or hardeners. Both groups are
currently considered as "fixatives". But in his time, especially
at the time of the first editions, the fixatives fixed, to avoid the deterioration
and loss of structures, and the hardeners (usually salts of heavy metals), were
those reagents that were able to give the tissues a proper hardness to allow it
to be manually cut into thin sections for microscopic study.
Yes, not only the
plant tissues were cut by hand! also the animal ones!
The appearance and
the rapid spread of mechanical microtomes, and the custom of giving consistency
to tissues through its inclusion in paraffin, erased, step by step, the
difference between fixing and hardening.
1/3 Alcohol (30%
more or less) was regarded as a fixative. Unlike the absolute alcohol it did not
produce "hardness", what did it useful only for certain organs.
Of course, using
the thin skin of onion, with only one cell thick, it is not a distinction that bothers
me. But it does matter to know the results of using it as a Fixer. I never used
as a fixative an alcohol of so low concentration.
Protocol:

Fig 8 - The simple and reduced series of
reagents
Fixing, 5 hours; water, 5 min.; Blue 1, 2
min; washing, rinsing
|
|
|
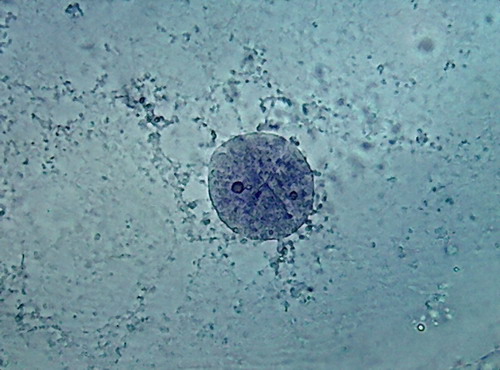
Fig 9 - 35%
Alcohol – 40x obj.
|
|
|
|
|
|
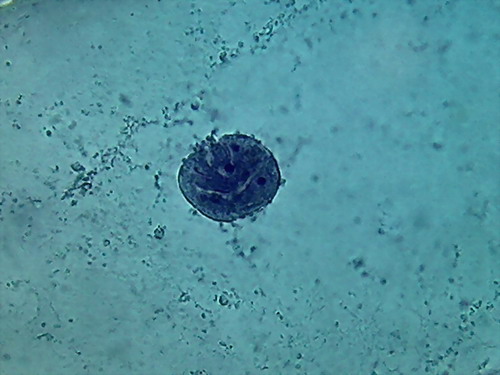
Fig 10
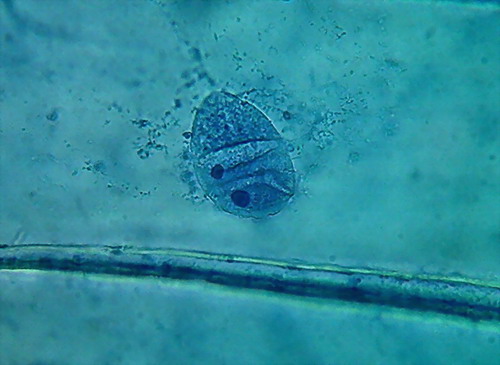
- 35% ALCOHOL – 100xOI – It is the second time, that I find a nucleus like the
one in the 3rd. image, when fixing with alcohol!!!
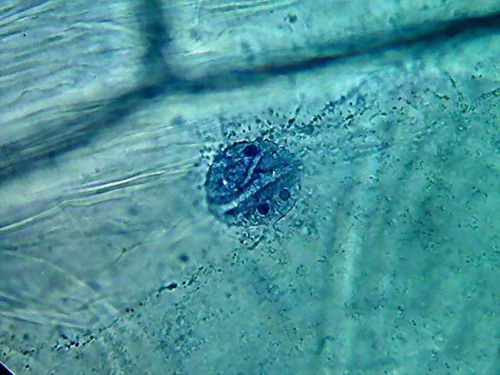
I tried 35%
alcohol, for what it is, a weak alcohol, which surely would need time to make a
complete fixation. And which could be dangerous, because it is not as good an
antibacterial agent as 70% alcohol. The pictures show a satisfactory nuclear
fixation, but poor cytoplasmic fixing, not very different, but inferior to
that obtained with 70% alcohol. And the intriguing punched nucleus!
A SHORT SUMMARY
If I
had to graduate the fixatives used until now to fix the onion skin, I would
only rule out the boiling water and the lemon juice. I think citric acid would
be a good option, but I would give 1% acetic acid or at most 2%, and to
96% alcohol, the best score as nuclear
fixatives. 70% alcohol produced nucleii as good as acetic acid, even if
some cells show the same deep grooves 96% alcohol show.
And I
would better classify 96% alcohol, and the old Iodine as cytoplasm fixatives. Review the pictures. Possibly all that is
needed is to stain the cytoplasm to make details more visible.
In the future, we will see...!
It is not worth
working with 35% alcohol, except for economic reasons, and only for educational
purposes.