Unsurprisingly, we human beings often interpret the objects and organisms around them in terms of models which we derive from our (sometimes misleading) understandings of ourselves and our experiences. A persistent and nagging question which arises regarding “simple” organisms is how they can produce astonishingly complex structures, demonstrate quite complicated behavior, and yet have only a rudimentary brain, if any, and a primitive nervous system. In part this perplexity arises because we have accepted a view of ourselves that assumes that the brain is in charge of everything we do, so we’re left with puzzles regarding how “brainless” beings can be structured the way they are and behave the way they do.
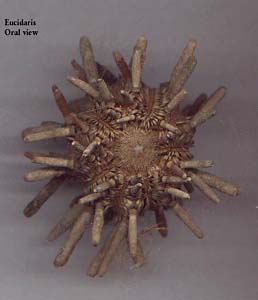
Sea urchin—Eucidaris, oral view.
Sea urchin—Strongylocentrotus droebachiensis.
Sea urchin— Test of Strongylocentrotus droebachiensis.Recently I started examining some sea urchins and, so far, I have been looking primarily at a species of Eucidaris, a tropical, club-spined echinoid, Arbacia—the “tooth pick” sea urchin, Heliocentrotus, Stronglyocentrotus purpatus and S. droebachiensis, so I will center my remarks around them. Virtually everyone who is interested in sea life has, at one time or another, seen either the spiny sphere that characterizes sea urchins or seen the test (“shell”) after the spines have been removed—an elegant and pleasing structure. If you look closely at the test, you will notice 5 double rows of miniature dome-like structures with a distinct mound in the center. These are the points at which the spines are attached and if you examine the base of a spine, you can see how these two structures form a ball and socket joint. These spines move both as a means of locomotion and as a defense against a perceived threat. How does the sea urchin “know” which direction to move in to find food or in which direction to move its spines when it “knows” it’s being attacked. The simple answer is that it doesn’t “know” any of these things. What we call knowledge is a human enterprise and even if we do someday encounter other “intelligent” species, it is virtually certain that their forms of knowledge will be significantly different than our own.

Arbacia - aboral view
Arbacia - oral view

HeliocentrotusThe explanation of what’s going on in the case of the sea urchin is enormously complex, and often when we’re faced with behavioral systems of such intricacy, we resort to reductively simple paradigms of explanation. To see how this works, let’s start with some “simpler” cases. Imagine that we are observing a large active amoeba; the movement of the pseudopodia is evident and we can also see the internal flow of protoplasm and the nucleus. In addition, we can watch feeding behavior—the flow of a pseudopodium around a prey organism, eventually enclosing it and forming a food vacuole in which the prey is digested. All of this seems rather straightforward and uncomplicated until we begin to ask certain questions:
1) Is the formation of and direction of movement in the pseudopodia random? Well, at first thunk (a “thunk” is a quickly and only partially formed thought), it would seem so. It’s certainly not like the hound picking up the scent of the hare and going rabbitting after it. With amoebae, there generally seem to be a need for a tactile encounter to produce the pursuit of prey. However, it is certainly likely that there are, in addition to the tactile responses, some other very complex biochemical and biophysical interactions going on.
There is a rather natural inclination to think of the nucleus as being something akin to a brain or, at least, a primitive coordinating center. The nucleus does indeed seem to act in concert with other structures in the organism, but there doesn’t seem to be much evidence that it is involved in locomotion or prey capture. Additionally, there is the problem of multinucleate protists, such as Pelomyxa which can have as many as 2000 nuclei and, since there doesn’t seem to be any network connecting them (other than the protoplasmic matrix), it is difficult to see how they could perform a coordinating function. In the first half of the 20th Century, there was considerable discussion regarding a “motorium” in ciliates which was conjectured to play a crucial role in the coordination of the ciliary waves in organisms, such as Paramecium. To my knowledge, the evidence for the existence of such a structure remains elusive. Some investigators claimed to have been able to demonstrate such a structure using fairly complicated staining techniques; others argued that the “structure” was an artifact of the techniques themselves. In any case, the existence of such a structure would certainly not provide an adequate basis for invoking an analog to the brain. In fact, it is our brain which produces the problem here, because we are so thoroughly accustomed to thinking of behavior as coordinated, directed, intentional, and “conscious.” If you ask: Is an amoeba conscious? the answer seems to be pretty clearly—No! However, if you ask: is an amoeba aware?—the answer is not so clear.
Here we are in a language neighborhood that is full of pitfalls and other subtle traps. If by “aware,” we mean that the organism responds more or less consistently to given stimuli and “behaves” in ways that can, after sufficient observation be predicted; then it seems reasonable to say that the amoeba is “aware.” This, however, is by no means the end of the matter; many philosophers, biologists, and psychologists have linked behavioral awareness to intention and purpose. Let us suppose that our amoeba forms a large pseudopodium which moves fairly rapidly. Can we describe this action as intentional? If there is nothing in that direction, the action seems random. However, if there is a prey organism in that direction, we might be more inclined to interpret this behavior as purposive. Nonetheless, this behavior may simply be the consequence of a series of complex biochemical and biophysical interactions; after all, much of our own behavior is just of that sort.
With many protists (among other creatures), much of their behavior does seem random and largely unpredictable. The slow, but persistent activity of amoebae provides them with enough food to maintain themselves. It should also be noted that some species are not dependent on active prey for food, but rather ingest algae and diatoms. On the other hand, highly active protists, such as, Paramecium, Halteria, and Urocentrum dart and whirl and pirouette, apparently ingesting almost anything that will pass through their cytostomes. They feed primarily on bacteria and very, very small protists and their environment, when they are flourishing in large numbers, is a rich organic soup. All they have to do is twiddle their cilia to get a gourmet repast.
The feeding behavior of Lacrymaria olor is both intriguing and frustrating. One can sit for half an hour watching the elegantly extensile “neck” and “head” strike out in all directions with no result. Its behavior rather reminds me of a paddle ball where the ball is attached to the paddle by means of an elastic string—the ball moves off at all sorts of different and unpredictable angles. Even when food organisms, such as Halteria, are present, there doesn’t’ seem to be any “targeting mechanism” in Lacrymaria, just random, blind strikes. However, once contact with a food organism is made, things change drastically. The trichites virtually instantaneously expand the opening of the cytostome and the Halteria is partially engulfed and forced down toward the substrate. Toxicysts may also be involved in the capture. The diameter of the Halteria is considerably larger than the diameter of the neck of Lacrymaria and as it moves down the neck toward a food vacuole, the appearance is rather like that of an ostrich swallowing a large grapefruit.
Protists have been ingenious in developing all sorts of clever traps for prey. Heliozoa have sticky flexible spines, suctoria have extensile tentacles with sticky disks at the ends, and foraminifera create elaborate protoplasmic nets. However, none of these devices for prey capture demand the concept of intention as an explanation of their function.
What we need is to find examples of stalking behavior in protists in order to examine the issue of intention. In a Paramecium culture containing Coleps, if a Paramecium is injured, the Coleps usually attack—6, 8, 10 of them, feeding like jackals. However this seems to be simply a biochemical trigger that activates the Coleps and we might even say that the behavior of the jackals is simply a more complex version of that which Coleps exhibits. Another voracious predator of Paramecium is Didinium nasutum which will swallow a Paramecium whole even though it’s larger than itself and it continues this behavior until the Paramecia have been depleted, forcing the Didinium to then go into a dormant state by encysting. Over the years, I have observed many very different types of amoeboid protozoans, flagellates, and ciliates and have noticed a wide variety of strategies for food capture; however, I can’t think of a single instance that would require a notion of intention or purposiveness to explain these behaviors. It simply doesn’t make sense to ask whether or not the amoeba “intended” to move in a particular direction at a given moment nor whether it “intended” to capture the Paramecium.
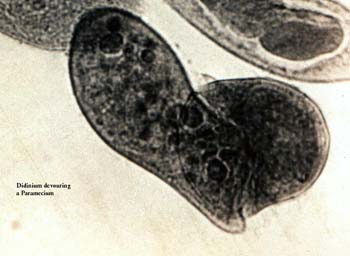
Didinium devouring a ParameciumAnyone who has spent any time closely observing large ciliates will almost assuredly have seem some of these organisms undergoing lysis (breaking open) as a consequence of the force of the pressure of the cover glass on them as the water gradually evaporates. [If you haven’t observed this phenomenon, it is easy to do so. Take a small drop of a rich culture of Paramecium, place a cover glass on it, and wait. You will see the Paramecia slow down, then become nearly non-motile, then flattened and completely non-motile. Shortly, thereafter, some of them will begin to lyse.] Protoplasm, vacuoles, vesicles, nuclei are more or less gradually emptied from the pellicle or envelope formerly enclosing the ciliate, but you will still be able to observe areas where the cilia continue to beat and do so until the organism is virtually empty of all components. To my mind this argues against the idea of a “motorium” or “neuromotor apparatus” or central coordinating organelle. It is certainly true that there are elaborate fibrillar systems in some ciliates. A system of fibrils ostensibly responsible for coordinating ciliary motion has been described along with the so-called silverline system which can be dramatically demonstrated using special staining techniques. The method used for this image was Protargol staining which is an organic silver compound. The technique is complex and tedious, but the results are very impressive. The relationship between these two fibrillar systems is still a matter of debate, although the discussion of a “motorium” or “neuromotor apparatus” has virtually disappeared from the literature in the last 50 years as regards protists.

BlepharismaThe classic work on a “motorium’ was done by Clark in the early 1900s. He centered his investigations on a truly remarkable and bizarre organism called Diplodinium (Epidinium) caudatum which is found as a symbiont in the gut of ruminants. Its extraordinary complexity—including tiny internal calcareous skeletal plates—led the distinguished invertebrate zoologist, Libbie Hyman, to remark that such organisms should be regarded as acellular rather than unicellular; that is, to describe such creatures as consisting of a cell or colony of cells is a serious misdescription; rather they are fully functional organisms in their own right. One might well be inclined to regard Clark’s work as revealing an anomaly in one organism or small group of organisms, were it not for the fact that a number of other investigators subsequently published reports of a “motorium” in a dozen or so other ciliates including free-living forms.
So, is the “motorium” an anomalous structure found in only a few organisms? If so, then how do we explain the ability of other ciliates to coordinate ciliary activity without a “motorium”? Perhaps the function of the “motorium” has been misdescribed or perhaps it is not truly a structure, but rather an artifact resulting from fixation or staining techniques or a combination of such factors. Here is a fascinating research project for someone—a survey of the literature from Clark’s work to the present and inquiries to prominent ciliatologists today regarding their views on the “motorium” or “neuromotor apparatus” could result in a valuable contribution. In any case, it is dubious that ciliates have some primitive analog to a brain and the use of the term “brain” to describe certain aggregates of cells in even higher groups of primitive invertebrates is a questionable matter, but that’s a topic for another essay.
Because we humans have large and complex brains that are capable of making choices, directing our behavior (or, at least, some of it) and devising strategies for dealing with variables in our physical and social environments, we consequently have a strong tendency to regard ourselves as having control of virtually every activity of our being as an organism and analogically we are inclined, as well, to think of other organisms as exhibiting similar, although lesser, forms of control and purposiveness. The more we learn about the complex behavior of any given organism, the more inclined we are to marvel at its interconnections and interactions with its environment, but we should not allow these factors to seduce us into a dubious leap toward teleology. We would not make that mistake regarding the formation of crystals and yet, they will form patterns according to certain rules and, if conditions change, they may alter their structure. Yet, in such cases, we would not think of raising the issue of “intention” or “purposiveness.” Design does not necessarily entail intention or purposiveness.If we watch a dragonfly nymph in an aquarium, we may very well observe what might be described as stalking behavior with regard to its prey. This, coupled with the remarkable compound eyes and it’s lightning-fast responses—just try to see that bizarre jaw structure snap out and seize its victim—may lead us to attribute “mental” processes to this organism in an entirely inappropriate fashion.
However, let’s go back to the sea urchins we started with. Interestingly, in terms of the phylogenetic tree, the sea urchin is generally placed at a “higher” level than the dragonfly. The reasons for this are very complicated, but we need to be careful not to be misled by these kinds of hierarchical charts of organization. The dragonfly has a brain, not a very big one, but certainly one sophisticated enough for it to cope with its environment in ways that make biotechnologists drool. Imagine being able to hover and dart and snatch mosquitos out of thin air—not the sort of thing one has time to “think” about; it’s simply done without any reflection. The sea urchin, although it doesn’t have a distinct “brain”, does have a remarkably extensive nervous network. There is a neural ring in the oral area with radial nerves, usually 5 or 10, branching off, and they extend around the entire digestive system. In addition, these radial nerves generate a nerve which goes up along the side of each tube foot and forms a neural layer on the disk end of the tube foot. Furthermore, these radial nerves also produce nerves that pass through the openings of the ambulacral plates and spread to form an elaborate neural network just below the surface of the skin. This means that each and every spine, pedicellaria, and tube foot is connected, directly or indirectly, to this neural system.
Remarkably, their sense organs seem very limited in variety, but are exceptionally abundant. The cells of the epidermis contain these sensory receptors which are sensitive to touch and, almost certainly, to a variety of biochemical stimuli as well. So, in effect, there are thousands of tiny sensory receptors and the tube feet, because of their extensility, play a crucial role in “tasting” the environment. What is clear is that echinoids have adapted extremely well to their environments and their means of orienting themselves, although limited, are highly effective. In one respect they sometimes exhibit behavior that creates a problem for them similar to one that we human beings have brought on ourselves—overpopulation. At times, under just the right conditions, the population of sea urchins in a kelp forest will explode and they will virtually destroy this forest which is their food source. Thereafter, of course, their population in that area declines dramatically, but in the meantime, they have done dramatic damage to that section of their ecosystem. There is a lesson here for human beings, but one which we seem reluctant to learn and, even worse, we can now affect not just our own local environment, but the balance of the entire planet. As I move toward senility, I become more and more convinced that much of our behavior is biologically determined and that our reason, of which we are so proud, in the end is often an ineffectual byproduct of a hypertrophied brain—but , I have such thoughts only when I’m in a highly optimistic mood.
However, all of this does hearken back to the initial question posed in the title: Who’s is charge here? It is evident that humans still have relatively little control over critical aspects of our world—volcanos, earthquakes, tornados, hurricanes and just plain old ordinary weather. Imagine if we could control just such basics as rain, snow, wind and temperature—we would see ourselves as gods! But, an even more fundamental question is: how much control do we have over ourselves as biological organisms? We certainly don’t want to think of ourselves as being like a sea urchin, a series of biochemical and biophysical reactions and responses to stimuli—complex, but “instinctual”, “mechanical” entities—for, after all, we have a brain and a very large and astonishingly complex one at that. Nonetheless, in the evolutionary development from a tiny nerve center, to clusters of nerves connected to sensory receptors, to complex nerve networks, to sophisticated brains, and finally to human brains capable of reflective consciousness, volition, and intentional activity—we are inclined to forget that, until the very last stage, these marvelous neural adaptations didn’t require any sort of direction or guidance. In other words, nerve networks, receptors, and brains in most organisms simply carry out their functions in a manner similar to a heart, a circulatory system, or a liver. This may seem a mechanistic and reductive view and, indeed, the British philosopher, Thomas Hobbes, proposed that consciousness is simply an epiphenomenon, a byproduct of the activity of matter or as he put it “the click and shimmer of atoms in the brain.”
If we stop and think about it, the vast majority of the activities which are involved in maintaining us as living organisms are carried out without any intercession on our part and the vast majority of biological activities are ones which we—sometimes to our dismay—have virtually no control over. There are times when if one overindulges, one would like to be able to give some very definite instructions to one’s stomach or liver, but, unfortunately, we are not constituted that way. An uncountable number of events ranging from the cellular level to the high brain functions are taking place in us every second and hardly any of them are under our direction or control in any sense of which we can be aware. How often do you think of your spleen or pancreas, or your big toe, except when you stub it or it gets ingrown; did you know that you have islets of Langerhans inside you and that this is not a vacation resort for lymphocytes? A few of you more mature readers (old), such as myself, may remember a rather inventive science fiction film called Fantastic Voyage—a film designed to demonstrate that the voluptuous Racquel Welch couldn’t act. But far more importantly, this was a film well ahead of its time. The special effects were and remain, remarkable (if not always accurate) in terms of giving us an idea of the extraordinary complexity of what’s going on inside the human body without any guidance from our consciousness, among other reasons because the human in question in the film has been anesthetized.
It is, perhaps, a blow to our egos to realize that there are “brainless” organisms which are, from a biological point of view, at least as successful as we are. However, perhaps even more disturbing to us is the recognition that not only are we not the “masters of the universe”, but as organisms we have little control over the fundamental biological processes that sustain our fragile consciousness. Three cheers for sea urchins!
All comments to the author Richard Howey are welcomed.
