
|
A Holiday Essay: Micro-Ornaments: A Do-It-Yourself Kit by Richard L. Howey, Wyoming, USA |
One needs to remember that only a minority, albeit a significant one, of the 6.5 billion people on this planet celebrate Christmas as a religious holiday. However, there are many more who celebrate it as an economic holiday–after all, who doesn’t like to get presents? So, I was wondering if I might through a bit of computer magic, come up with some Christmas tree ornaments using images I’ve taken through the microscope. If you like them you’re welcome to print them on nice heavy paper, cut them out, and hang them on your tree. If you don’t like them, perhaps you can figure out how to enlarge them a bit, print them, cut them out and glue them to your dart board.
Usually my essays ramble on and on, but this is a busy time of the year and I don’t have time to write a long essay and you don’t have time to read one, so I’ll just present 12 images for the 12 days of Christmas (and the thumbnails to show you how they started out) and only give you very brief descriptions of what they are.


The crystal was formed from a mixture of magnesium chloride and a drop of a solution of a biological fluorescent stain known as DAPI. Polarized light.


This rather unprepossessing mixture of minute crystals was a combination of magnesium sulfate and a very dilute solution of ascorbic acid (Vitamin C), but with a little computer manipulation, it produced a quite lovely ornament. Polarized light.
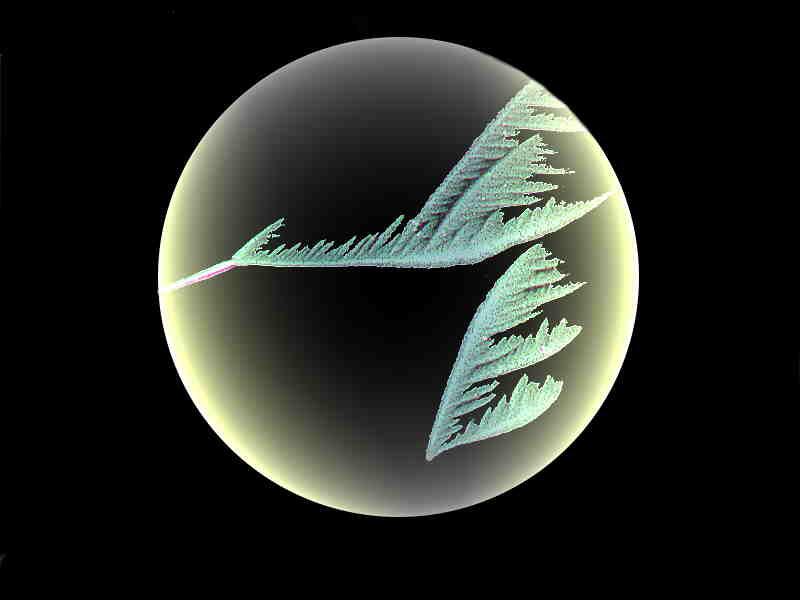

This image might look familiar to you from last month’s gallery. The crane-like crystal was produced by mixing Bromoseltzer, sodium silicate, and the red dye Alizarin Red S. Polarized light.
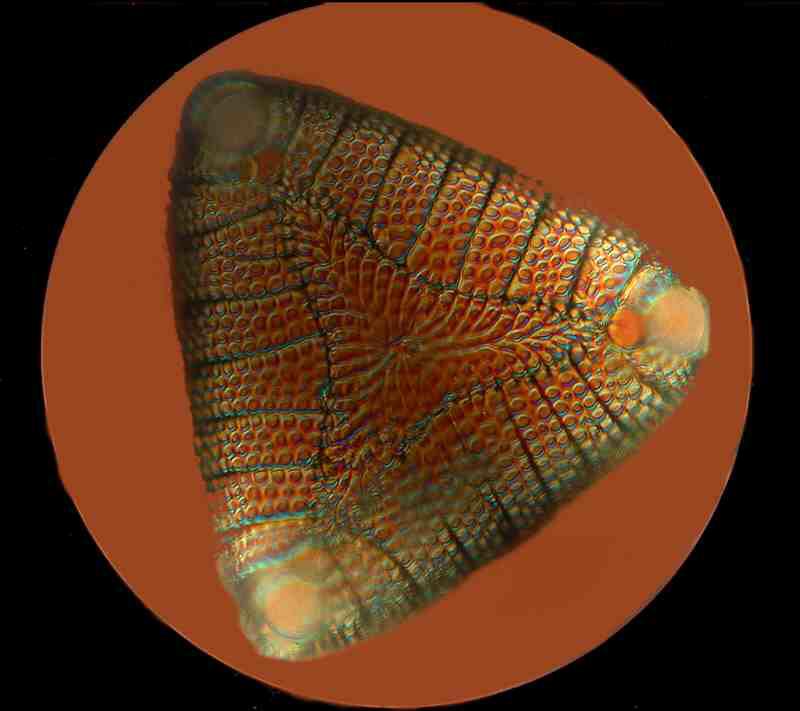

This is a beautiful diatom, which is the siliceous (or glass-like) shell of a tiny protist. It was photographed using a special technique called Nomarski differential interference contrast which accounts for the color, since special prisms can be adjusted to produce what is known as “optical staining”.
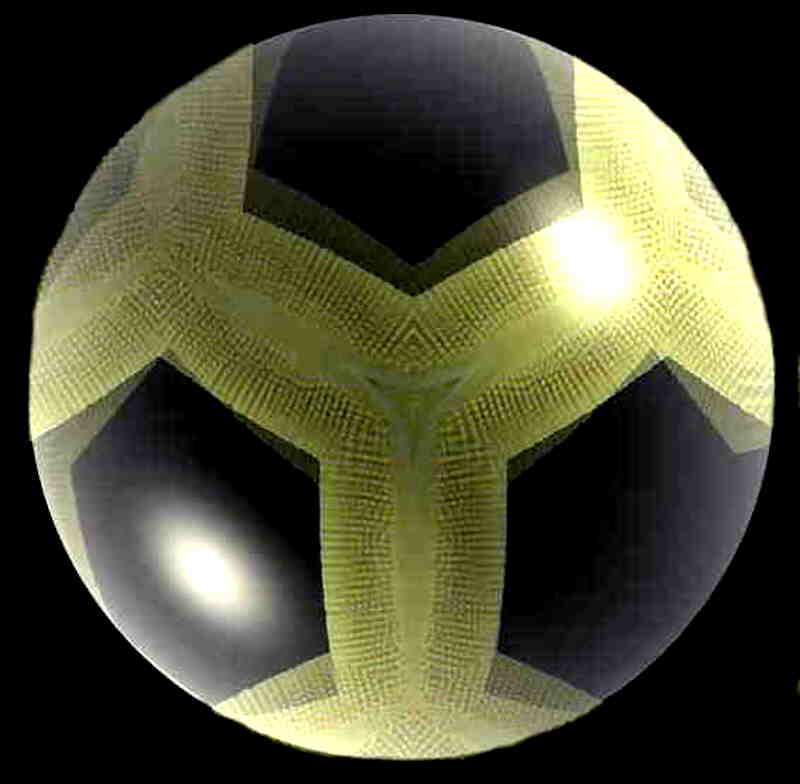

Another more common type of diatom. Nomarski differential interference contrast.
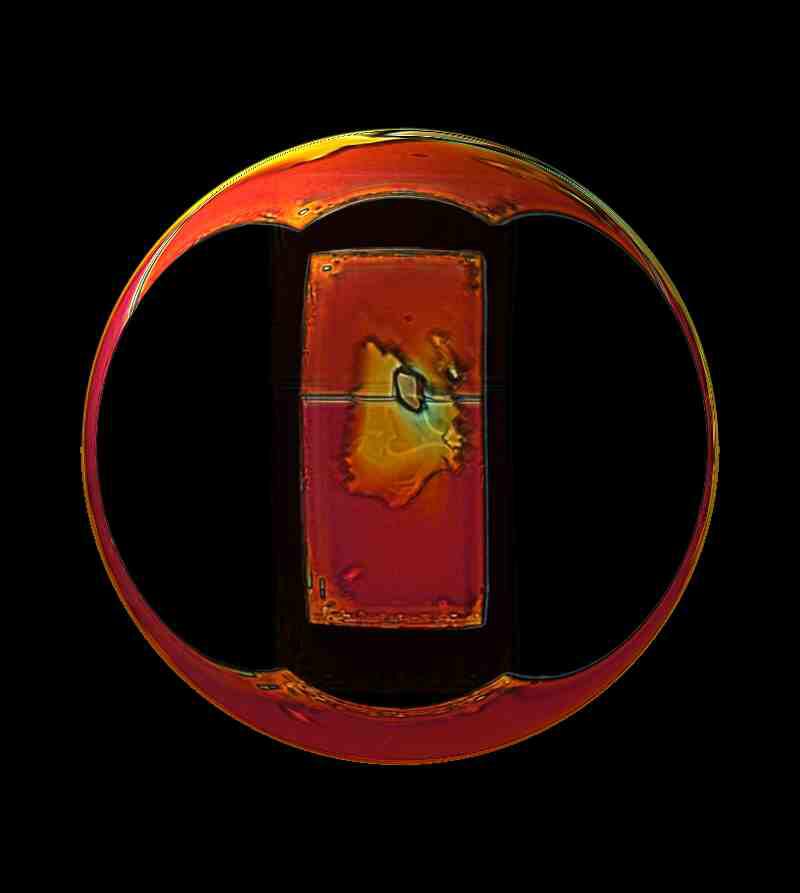

Sometimes the use of ordinary household products can produce some surprising results. Again this is magnesium chloride, but this time mixed with a bit of Elmer’s Gel Glue. Polarized light.


Yet once again, magnesium chloride, only this time mixed with a bit of the biological stain Crystal Violet. Polarized light.


A common household pain reliever, acetaminophen, combined with a bit of nickel sulfate, a substance sometimes used to anesthetize protozoa. Polarized light.
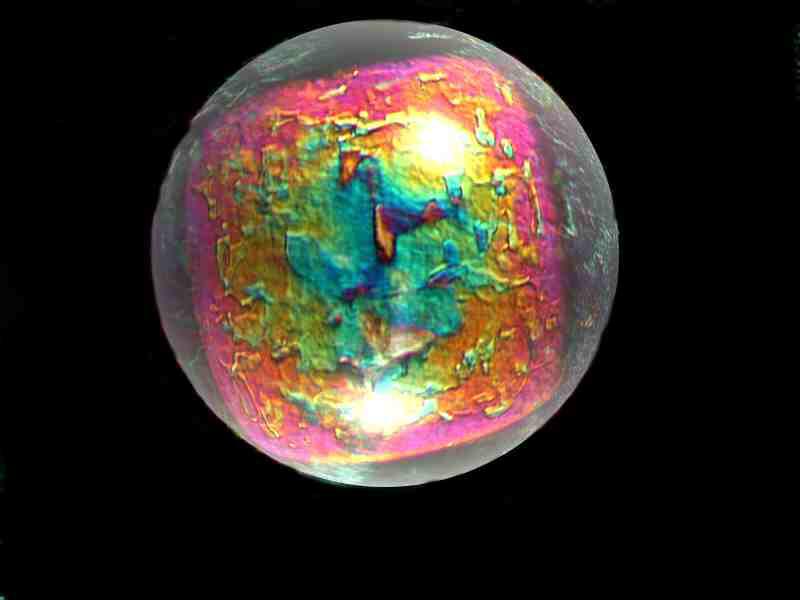

A drop of the toothache medication, Anbesol, combined with magnesium chloride produces a colorful display. Polarized light.
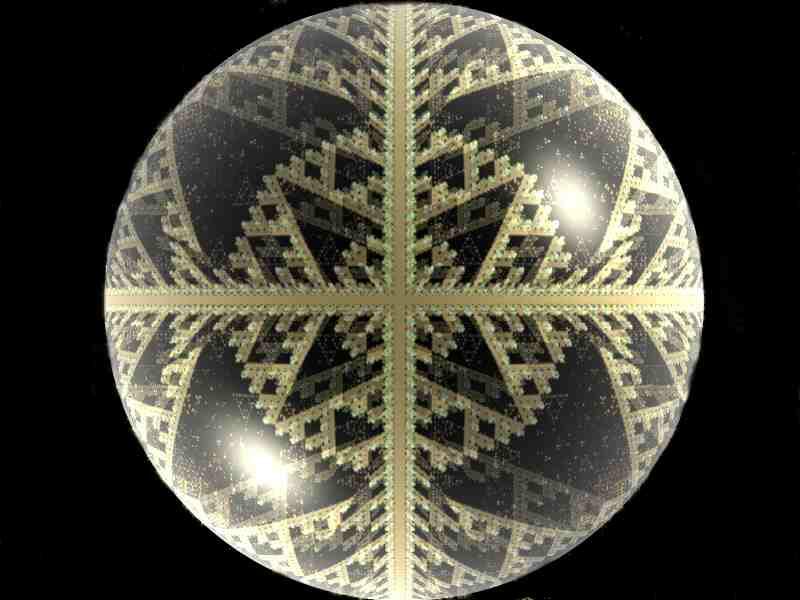
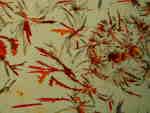
The biological stain Orange G produces a variety of wonderful effects under polarized light and the colors with change significantly as one rotates either the polarizer or the analyzer.
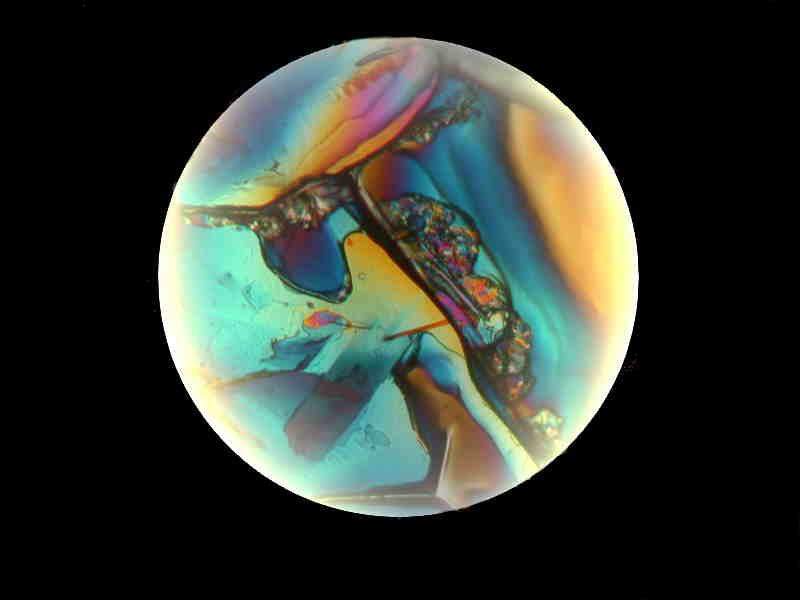

Again, a bit of Orange G, but this time mixed with some sodium bicarbonate, and a bit of borax.
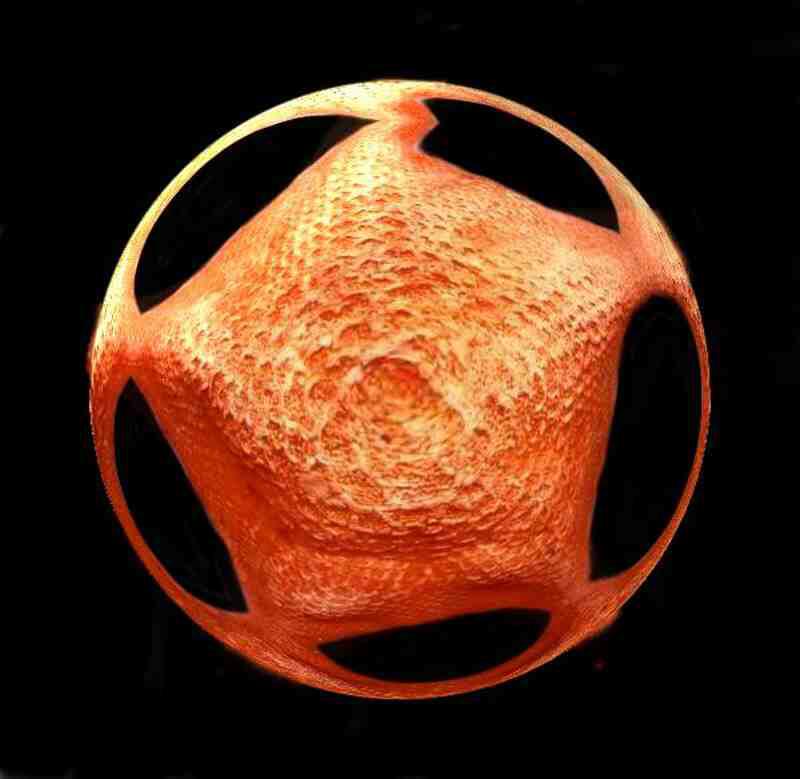
This one isn’t really microscopic. It was an image which I took of a bat starfish, but I liked it enough that I thought that I would include it anyway.
As you can see from this little gallery the possibilities for the computer manipulation of images is endless, so don’t ever buy a used car from a microscopist.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK