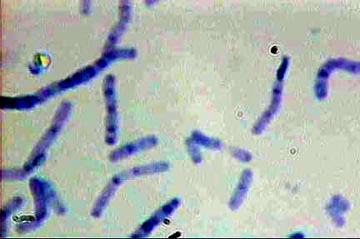
Bacilli - Loeffler's Methylene Blue
|
Looking at Bacteria by Mike Morgan, UK |
Introduction
Looking at pond water under the high power of the microscope often shows minute organisms of various shapes moving in the background.
More than likely these are bacteria.
There are three main classes of bacteria:
1. A straight rod called a Bacillus, plural Bacilli
2. Spherical called a Coccus, pleural Cocci
3. Curved or spiral called a Spirillum, pleural Spirilla.
Bacilli are the commonest forms of bacteria.
Cocci are small spherical organisms which may be seen in pairs, when they are referred to as ‘Diplococci‘.
Sometimes the Cocci form long chains and are then known as ‘Streptococci’.
If the Cocci form groups, like a bunch of grapes, they are called ‘Staphylococci’.
A Spirillum may appear as a single curve, looking like a comma, when it is referred to as a ‘Vibrio’.
A bacterium which has the appearance of a corkscrew and is quite long is known as a ‘Spirillum’.
Observation
In order to observe the bacteria, more clearly, it is necessary to make smears of the bacteria on microscope slides. The smears are then stained to make the organisms more visible.
The most important stain in bacteriological examinations is Gram’s stain, which was first described some 120 years ago.
Using Gram’s stain, the bacteria are classed as Gram-positive or Gram-negative. Gram-positive bacteria stain blue-black and Gram-negative bacteria stain red.
Gram-positive bacteria have a mesh-like cell wall made of peptidoglycan, capable of retaining the violet dye used in the Gram staining.
Gram-negative bacteria have a thin cell wall made of a layer of peptidoglycan, surrounded by an outer membrane containing lipids. Gram-negative bacteria are decolourised in the procedure. To visualise Gram-negative counterstain with safranin.
Experimental Procedures
1. Bacteria in pond water:
Melt some nutrient agar and add a few drops of water with a sterile pipette. Mix and pour into a sterile petri dish. Leave at room temperature for a few days and examine the colonies of bacteria.
2. Bacillus mesentericus:
Place a slice of potato into a 300 ml beaker of water. Leave for several days and then remove a drop of liquid from the water surface with a pipette. Place on a slide and examine under the high power.
This will show the rod shaped B. mesentericus. The preparation may be stained with iodine by irrigation.
3. Bacillus subtilis:
Prepare an infusion of hay by pouring hot water on to some hay. Leave to stand for a few days. Filter the infusion and place a drop of the liquid on a microscope slide. Examine, as before, under the high power.
Note: It is worthwhile, with the both the potato and hay infusion, to spend some time examining the liquid for various protozoa, which will have become established.
Smear preparations
To make a stained preparation of the bacteria a spread of the bacterial cells is made on a microscope slide. Fixation of the cells is provided by heat.
Technique
1. Heat a slide over a small flame to remove any grease.
2. Sterilise an inoculating loop in the flame and use this to transfer a loop full of sterile water to the centre of the slide.
3. Sterilise the loop, or a needle, and collect an amount of bacterial growth and emulsify on the slide.
4. Dry the film over a small flame and fix by passing through the flame a few times. Do not overheat. The cells will now be adhered to the slide and the smear is now ready to be stained.
Simple Stains
General stains that can be used are Ziehl’s carbol-fuchsin or Loeffler’s methylene blue.
Carbol Fuchsin (Ziehl-Neelsen)
Basic fuchsin 1 g
Phenol 5 g
Alcohol 10 ml
Water 100 ml
Dissolve fuchsin in alcohol: dissolve phenol in water and mix the two solutions.
For use the carbol-fuchsin is diluted x 10 with water.
Stain for ½ minute.
Loeffler’s Methylene Blue
Methylene blue 0.5 g
1% potassium hydroxide solution 1.0 ml
Alcohol 30 ml
Water 100 ml
Warm the water to about 50° C, stir in the methylene blue and add the other reagents. Filter.
Stain for 5 minutes.
With either stain the excess stain is washed off with tap water, the slide is drained, blotted and completely dried by waving over a small flame.
An oil immersion objective must be used to examine the stained smear.

Bacilli
- Loeffler's Methylene Blue
Staining with Gram’s Stain
Reagents:
1% methyl violet 6B
Lugol’s iodine (Iodine 1 g; potassium iodide 2 g; water 100 ml. Add iodine and iodide to about 25 ml water and when solution is complete add remainder of the water)
Dilute carbol fuchsin
Acetone
Method:
1. Fix the smear, as previously explained.
2. Stain with methyl violet 6B for one minute
3. Wash off the slide with Lugol’s iodine and flood slide with fresh iodine for one minute
4. Differentiate rapidly with acetone
5. Wash with tap water
6. Counterstain with dilute fuchsin for 30 seconds and blot dry.
Examine with oil immersion.
Staining Reaction:
Gram-positive organisms … blue-black
Gram-negative organisms …red
Cell nuclei …………………red
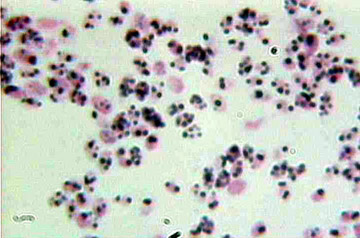
Gram
positive cocci
Safety Precautions
Any micro-organism must be treated as a potential pathogen and simple rules should be followed to ensure safety.
1. Ensure cuts and abrasions on hands are covered with a dressing before handling cultures.
2. Avoid eating, smoking and licking labels, when dealing with cultures.
3. Never pipette by mouth, always use a teat pipette for transfer of culture liquids.
N.B. Do not forcefully squeeze the liquid from the pipette, as an aerosol will be created.
4. Used cultures and microscope slides should be quickly disposed of as they are a potential hazard. These should be placed in a disinfectant solution after use.
5. Ensure hands are thoroughly washed and dried at the end of any experiment.
6. All chemicals used in the preparation of the above stains are hazardous and gloves and safety goggles should be worn when these are being prepared.
Suppliers:
Nutrient Agar: Blades Biological (01342) 850242
References:
Biology Staining Schedules R.R.Fowell ninth edition.
Biological Staining Methods George T.Gurr seventh edition.
All comments to the author Mike Morgan are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK