
|
Wild Cultures and Subcultures: Part II (with a small gallery of protists at the end) by Richard L. Howey, Wyoming, USA |
Last month we looked at some issues regarding cultures and this month I want to focus on subculturing.
Subculturing, although it can be time-consuming, is one of the more satisfying undertakings of the aquatic biologist, once you get the hang of it.
1) When To Subculture
This should always be done at 3:27 a.m.–sorry, but it’s summer break and I don’t have any students to torment with my bad jokes right now. Actually, timing subculturing is rather easy to determine and falls into 2 major categories.
a) When a culture reaches its peak and the organisms are abundant and flourishing–this is an ideal time to subculture. Check your notes and reproduce the conditions of the initial culture as closely as possible. If it’s an organism which is unusual and/or of special interest, you may wish to make several subcultures to make sure that you maintain it in abundance.
b) When a culture is limping along, but you, nonetheless, have sufficient organisms to experiment with subculturing, do so. Try starting a few subcultures by varying the media and keeping careful records. If one of them succeeds, you can go back to the usual form of subculturing described above.
2) Which Organisms To Subculture
Establish some clear priorities. Organisms such as Paramecium, Chilomonas, Halteria, Colpidium, Colpoda, Spirogyra, Vorticella, Oscillatoria, etc. are all usually readily available, easily maintained and, as a consequence, the loss of such cultures is not particularly problematic, since they are easily re-established. However, if you have the means, you can have one of your many laboratory assistants maintain stock culture for you, and lacking such an assistant, you can probably manage it yourself, since the organisms require relatively infrequent subculturing. It is a good idea to always have a stock of Paramecia on hand as it makes a good test organism and Chilomonas is also important since it often makes a good food organism.
I’ll just mention a few of the kinds of organisms which I have made efforts to cultivate with varying degrees of success.
a) First of all, almost any unusual organisms and especially those which I have not encountered before, I try to culture, be it an algal form, a protozoan, a lovely aquatic flatworm, a burgundy-colored cyclops, a strange cladoceran or the bizarrely wonderful Chaoborus midge larva, that has special little oval-shaped organs which function like air tanks on a submarine to control its buoyancy. It’s fascinating to watch them in a jar or small aquarium where they can hover motionless and then slowly sink or raise themselves toward the surface. When they find prey, their fierce jaws snap out and seize the victim faster than you can see.
b) The large ciliates and a number of the mid-sized ones are especially intriguing to me.
b1) Stentor coeruleus, the large blue Stentor is fairly readily cultured and is a magnificent creature to observe. In older neglected cultures, it tends to “monster formation.” There are a number of other small Stentor species, almost all of which are relatively easily cultured.
b2) Bursaria truncatella is like an enormous (micro-world-wise) crystal Art Deco vase. It swims through the water in a stately fashion and has an exceptionally large, spiral cytostome which allows it to easily engulf Paramecia. It’s a bit tricky to culture for more than a week or so. If you want keep it going longer, you will need to keep it in a relatively “clean” culture with sufficient food, but not too much. If it has too little or too much in the way of food, it tends either to encyst or disintegrate. It is essential to avoid high levels of bacterial growth and frequent subculturing is recommended.
b3) Dileptus anser is a wonderful beastie whose anterior end, extending up from the cytostome, waves through the water like the trunk of an elephant. Its needs in culture are similar to those of Bursaria, except that smaller food organisms, such as Colpidium, rather than Paramecium, should be provided.
b4) Another of the giants of the protozoan world is Spirostomum ambiguum, a long, band-shaped, highly contractile ciliate. Different species of Spirostomum vary widely in size, but S. ambiguum is well over 1,000 microns when extended and can get up to 3,000 microns. When disturbed, it does its magic act and contracts to about 1/3 its previous length. It is also of interest because of its long chain nucleus and an immense posterior contractile vacuole. I have described Spirostomum as micro-whales, since they are the leviathans of the micro-world and feed largely on bacteria which is parallel to many of the great cetaceans feeding on plankton.
I have kept long-term cultures (several years) of 3 different species: the giant S. ambiguum, S. minus which is not quite so long and much narrower and with a shorter chain nucleus, and S. teres, a rather small species (150 to 200 microns) with a single ovoid nucleus. The matter of contractility alone makes them well worth studying.
b5) Blepharisma, like Stentor coeruleus, has a distinctive pigment, only in this case, it is a pink to light rose color. The pigment is unique and is called blepharismin. It is a photoactive pigment and acts as a biochemical signal; if Blepharisma is exposed to strong light for a prolonged period, the pigment becomes toxic to the organism and eventually lethal–something to remember when you are examining this beastie for long periods under the intense illumination of the microscope.
All of these large ciliates culture reasonably well in Giese salts with either a grain of wheat or rice added.
Other ciliates have rather special dining habits and need to be “spoon-fed” as it were. Didinium nasutum is probably the most common example and feeds almost exclusively on Paramecia. I have read a number of accounts which inform me that when the Paramecium population has seriously diminished that the Didinium will begin to form cysts and that all one has to do to revive a culture is add a fresh supply of Paramecia–or that if you have let the culture go until only Didinium cysts remain, that you can keep them in water, add Paramecia months later and the Didinium will excyst and produce a flourishing culture. To this I emphatically say–Nonsense! Over the years, and as recently as last month, I have tried this at least a dozen times with no success. I mention this, because sometimes, despite your best efforts, an organism may frustrate your attempts while seemingly everyone else has great success. A late colleague of mine, who was a distinguished cell biologist and an exceptionally able and astute protozoologist, asked me to provide him some specimens of Lacrymaria olor, which I did gladly–on quite a number of occasions. Despite the fact that I had published a protocol for culturing this particular organism and, that a fair number of people in other laboratories in various places around the country had used it successfully, he was never able to establish stable cultures in his lab. Secretly, I always suspected that his graduate assistants were to blame. Here, the message is, don’t get frustrated if you can’t succeed with a particular organism. Micro-environments are exceedingly complex. Today I was looking at a depleted Lacrymaria culture and discovered some bacterial colonies unlike any I had seen before. Each had a center point from which emerged a balloon-like structure and from the base a series of “spokes” radiating out like a line of spaced beads which got smaller and smaller as their distance from the center increased. So, that culture failed Lacrymaria-wise, but certainly succeeded in other respects.
c) Large and medium-sized amoebae can be fairly readily subcultured and kept thriving for months. It is advisable to use a salt solution which you can make up in your own lab if you have the chemicals and a good balance with a sensitivity to 0.01 gm. Alternatively, you can buy amoeba medium from a biological supply house. In any case, you can add a grain or two of wheat or rice or a few pieces of boiled hay. When I have time I like to use all three in separate dishes. Right now I have 4 subcultures of Amoeba proteus going and one of them (a rice/Giese salt culture) I have intentionally rather neglected. Amoeba proteus is a magnificently strange organism and when neglected manifests an astonishing rage of intriguing morphological variations. This particular culture is certainly flourishing in terms of sheer numbers, for there are hundreds of specimens in my little 2 inch dish. Some look just like you would expect any self-respecting Amoeba proteus to look, whereas others are hardly recognizable. Some have been feeding in a irregular manner and appear quite black at 60x under my stereo microscope. Others have ceased extending pseudopodia altogether and have contracted into a spherical blob containing an enormous vacuole. Yet others are typically extending just one long pseudopodium which is atypical behavior for Amoeba proteus, but usual for a mature Pelomyxa. I would like to photograph or videotape these many morphological variations, but again I remind you that amoebae are (especially large ones) dramatically 3-dimensional–put them under a cover glass and there’s a good chance that you’ll crush most, if not all of them. So, what I hope to do is start a culture in a special chamber for my inverted microscope, so that I can observe and, it is hoped, photograph them without having to remove them from the environment of their culture.
d) Small amoebae and heliozoa sometimes show up in enormous numbers in cultures of other organisms. This makes them fairly easy to subculture if you get interested in studying them. They have some very unusual characteristics and one can spend a great deal of time investigating both their morphology and their behavior.
e) Medium-size ciliates. Very small ciliates often move exceptionally rapidly, which along with their size, makes them very difficult to study, but there are a number of interesting ciliates of intermediate size which are readily cultured and can be maintained in subculture with not much effort. Many of them do quite nicely in rather rank, neglected cultures. Hypotrichs thrive in a variety of conditions and are thus quite adaptable. They are of special interest because of their special organelles called “cirri” which are fused bundles of cilia creating a thicker, stouter structure than the traditional cilium. They often use their cirri to walk or scamper across the bottom while feeding.
Another ciliate which shows up very frequently in my old samples and cultures is Cyclidium glaucoma. This organism has long caudal cilia rather like bristles and an undulating membrane around the cytostome. Its behavior is both fascinating and frustrating. It will sit motionless on the substrate, sometimes for minutes at a time and then just at the moment when you rotate you turret to examine it with a higher powered objective, it bounces off out of the field of view as though it were spring loaded. Halteria grandinella is also a leaper.
Coleps is at times quite common and will attack injured larger organisms such as Paramecia. They are barrel-shaped, some have 4 or 8 spines toward the posterior end, and they are armor-plated.
Colpoda can almost always be found by taking a sample of good soil, add a salt solution, and a grain of wheat. These organisms are roughly kidney-bean shaped and are voracious feeders on bacteria and minute protists.
The aptly named Urocentrum turbo whizzes through the field of view with such speed that only the most indolent observer can ignore it. Then suddenly one will come to a screeching halt, attach to the substrate with a bit of “glue” from its tuft of longish caudal cilia and proceed to spin like a top. It is marvelous to behold.
f) Flagellates. Personally, I find trying to culture the more interesting flagellates rather frustrating. Balanced salt media are important along with low bacterial growth, plus a proper balance of light, temperature, and oxygen. Volvox are among the most lovely and astonishing denizens of the micro-world; they look like great crystal spheres rolling through the water and when they occur in great numbers, they tint the water a delicate shade of green. They consist of hundreds of cells and frequently have subcolonies growing in them. There is an intricate lattice of connection between the cells which is clearly visible at high magnifications. They obtain nutrition photosynthetically, thus the green tint. In general, I have not had good luck with trying to culture, let alone subculture, chlorophyll-bearing flagellates. However, I have been able to maintain phytoflagellates for periods of 2 to 3 week and this provides sufficient time to do some intensive examination. Smaller colonial flagellates, such as, Pandorina, Gonium, and Synura are certainly worth attention as well.
There are a number of interesting species of “armored” flagellates or dinoflagellates in fresh water, but the marine species are of special concern. Sometimes both in lakes and ponds as well as the ocean, algal “blooms” will occur and a few species will show up in uncountable numbers. These are most serious in marine coastal environments. Since certain dinoflagellates contain a powerful toxin when shellfish and other filter feeders ingest them, they can become toxic, even fatal, to humans who eat them. The dinoflagellates which occur in the lakes and ponds here in the high plains and mountains seem to prefer quite cold water and thus are not easily cultured.
Euglenoids are somewhat easier to culture and a medium made from split peas is frequently used for them.
Two brief sets of final remarks. Whenever you find an organism that’s of interest and which is thriving–subculture it! I am all too aware of the fact that this can quickly get out of hand without having a separate room for maintaining cultures with a variety of climate control features and a laboratory technician to oversee all of this, preferably one who will do all of this work on a volunteer basis because he or she is dedicated to science rather than that awful stuff we call money. However, until Microsoft establishes the Foundation for the Encouragement of Amateur Microscopy and offers grants to supply us with equipment and technicians, we shall just have to muddle along as best we can and if one looks at Micscape, I think we have proved that indeed we can muddle along, but a bit of financial encouragement would certainly be welcome. Perhaps we need to hire some creative accountants.
My absolute final remarks here have to do with a critical issue which I haven’t yet broached–glassware (and plasticware). Never use glassware which has contained potential toxic chemicals either for culturing or for the preparation of culture media. Glass is an extraordinary substance and, in spite of its hardness and toughness, it is easily contaminated at the micro-level. Anyone who employs any fixatives using mercury salts, such as Schaudinn’s fluid, should use disposable glass- and plasticware. Only amateurs who have extensive experience in handling such dangerous chemicals should use such fixatives at all. Not only will mercury salts contaminate glassware, they are extremely poisonous. So, whatever chemicals you use in your lab, keep all glassware used for reagents completely separate from dishes, beakers, flasks, or pipets used for culture or the preparation of culture media. Soaps, detergents, and even alcohols can leave fine films or deposits and some of these may have adverse affects on the organisms you are trying to culture. Cleanliness is next to godliness—but not always. I would rather risk a few organic contaminants from old cultures than try to figure out whether a soap, detergent or alcohol is creating a problem with my culottes.
So, my procedure is not very scientific, rather casual, and a bit sloppy, but is has worked rather well for me for quite some years. I have only a small bathroom sink for cleaning my culture dishes, so when I get enough to fill the basin, I take a small plastic knife of the sort one takes along on a picnic and I scrape out any excess detritus, dried wheat or rice grains. I fill the basin with very hot water and let the dishes soak for 1 or 2 hours. After that, a medium-sized, natural bristle brush can be used to do a final cleaning of the inside of the dishes. The brush should be well rinsed and not used for any other purpose. There are 3 drying possibilities which I use. When I have a bit of space and things aren’t too cluttered (which is very rare), I simply spread the dishes out and let them air dry. Sometimes I dry them with paper towels, preferably cheap, plain brown ones that haven’t been treated with bleaches or dioxin or I will use a soft kitchen towel of the sort that you used to use for drying dishes before you got your dishwasher.
Several years ago, I came across a super sale of plastic bottles . (I’m a total sucker for sales. One of my friends claims that I would buy typewriter ribbons if they were on super sale, even though I no longer own a typewriter. I think that’s a bit of an exaggeration, but I do admit to having made some strange purchases.) The bottles were 8 ounces and had plastic screw cap lids–quite suitable for collecting pond samples, so I bought 50 of them. These were 20 cents each, so for the modest investment of 10 dollars, I have enough to last me the rest of my life, since I use and reuse them. I also never wash them. When I’m finished with a sample, I either let it dry up or if it’s a smelly one, empty it out in they alley and then let it dry in a box which I keep in the garage for sample bottles. The next time I go collecting, I take a twig and scrape out any excess, encrusting debris, fill the jar with water, shake it vigorously, empty it and collect a new sample. If there are some leftover cysts that insist on excysting after such a long period of encystment, well–so be it.
A Small Gallery of Protists
1. Spirogyra. A common and lovely filamentous alga.

2. Amoeba proteus. Perhaps the most famous of all amoebae and yet it is not that common in samples. Fortunately, it is easily cultured and can be obtained from many biological supply houses. This specimen came from a reservoir about 25 miles east of Laramie.

3. Colpidium sp. This is also a very common organism and cultures readily. It is important because it can be used as a food organism for many other larger ciliates. The color here is a consequence of Nomarski differential interference contrast.
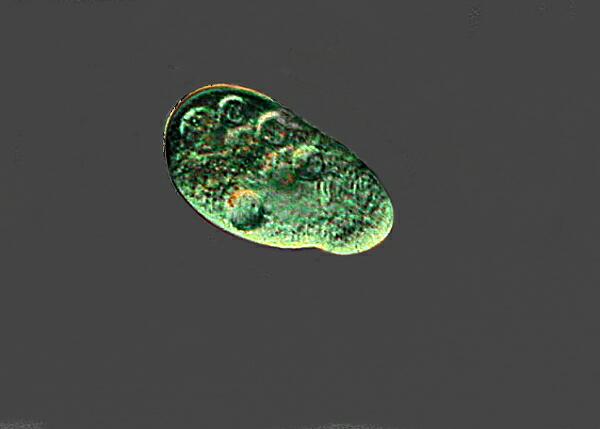
4. Didinium nasutum. One of the most voracious ciliates. These images were taken from a prepared slide. This first image is of a single Didinium up close showing the distinct macronucleus, the two distinctive ciliary bands which run around its circumference, and the prominent proboscis which contains a series of structures called pharyngeal rods. These rods allow the cytostome (mouth) to expand to a remarkable degree.
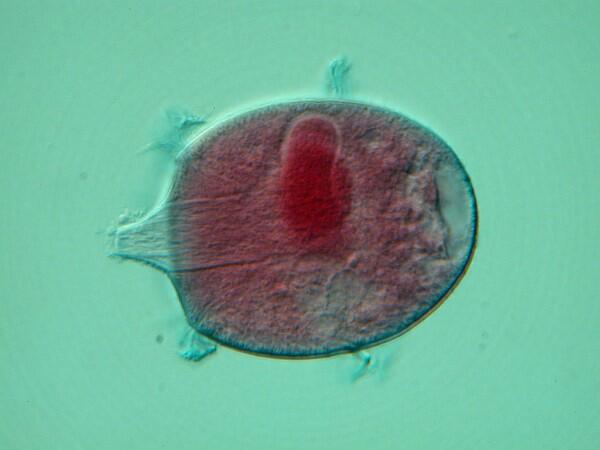
5. Didinium attacking Paramecium. Here we see Didinium ferociously attacking a Paramecium and as you can see the mouth has partially expanded.
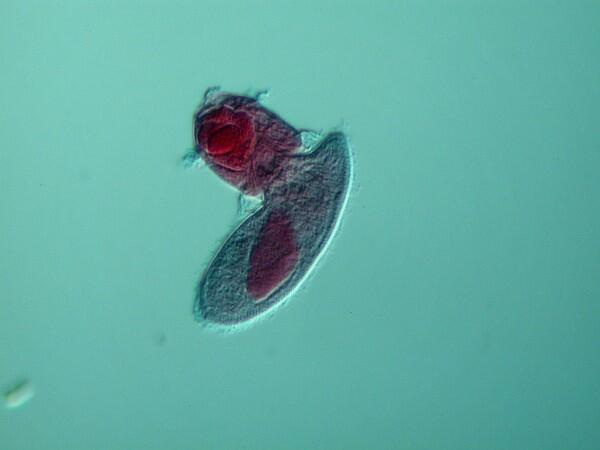
6. Didinium devouring Paramecium. As you can see, Didinium is able to expand its mouth to such an extent that in can engulf an entire Paramecium.
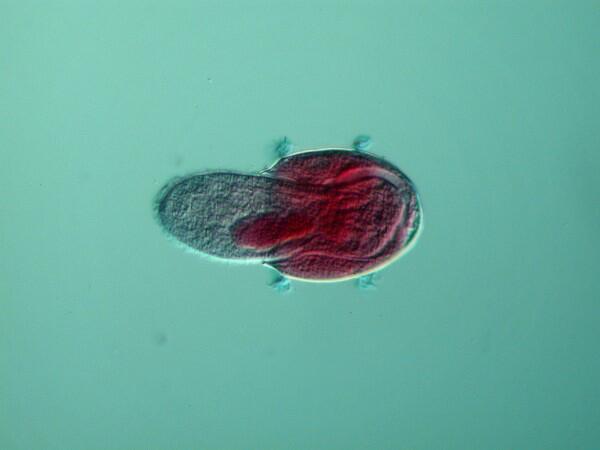
7. Dileptus anser. This bizarre ciliate waves its “trunk” rather like an elephant and at the base of the trunk is the cytostome which has a grouping of toxicysts which it can discharge to stun potential prey.

8. Spirostomum minus. This is one of the species of Spirostomum which I have elsewhere described as “micro-whales”. This specimen was approximately 1200 microns in length. It is highly contractile and has a beaded macronucleus.

9. Spirostomum minus. I have stained this specimen causing it to contract and the stain (Methyl Green Acetic) shows the beaded macronucleus clearly.
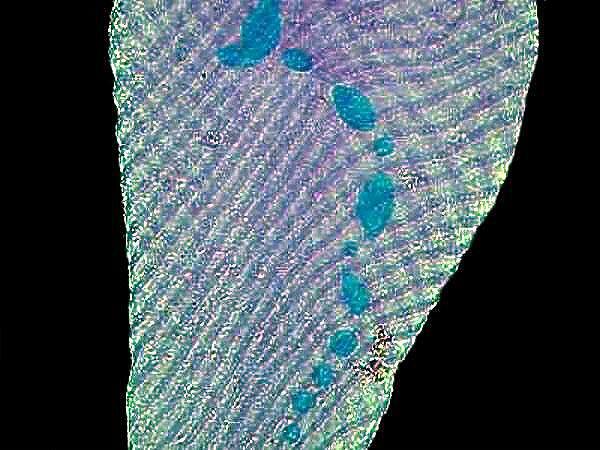
10. Vorticella sp. Like Spirostomum and Stentor, Vorticella is contractile. This lovely bell-shaped organism was first described by Leeuwenhoek. The bell itself is contractile and there is a long fiber called a myoneme running through the stalk, as you can see in the image. When disturbed, the organism contracts and the stalk coils up, then slowly uncoils later.
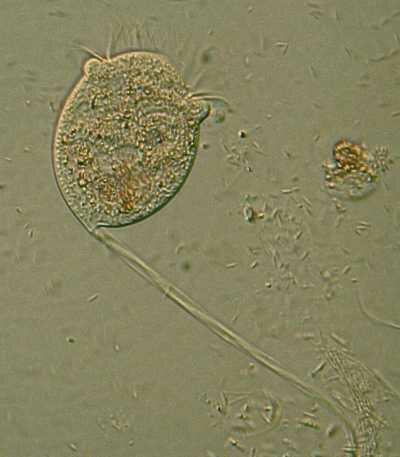
11. Stentor coeruleus. This species has a distinctive dichroic pigment which usually gives it a blue-green appearance, however, when the light shifts to just the right angle, the organism takes on a rose-colored tint. This specimen was swimming and is not fully extended.
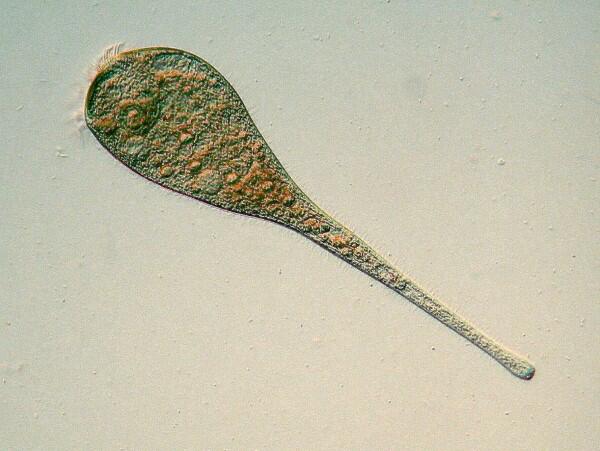
12. Paramecium. Along with Amoeba proteus, perhaps the most famous of all the protozoans, Paramecium is anything but a typical ciliate. It has many fascinating morphological features, is easy to culture, and is an excellent organism for conducting a wide variety of experiments.
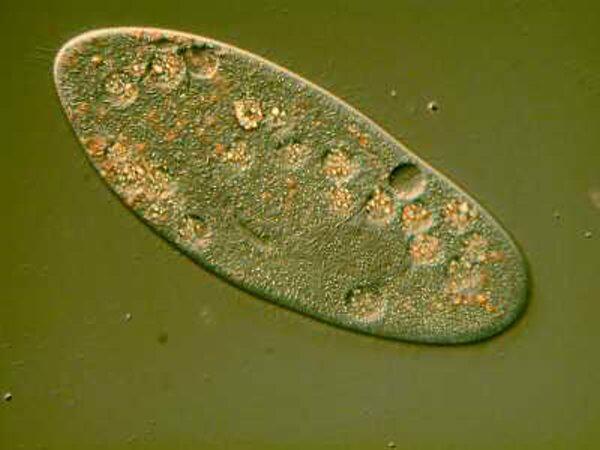
13. Thecamoeba. These remarkable creatures have a textured membrane, are often found in cultures made from soil taken from potted plants, and have both unusual morphological and behavioral characteristics. You can see that it has ingested a rotifer and some filamentous algae.
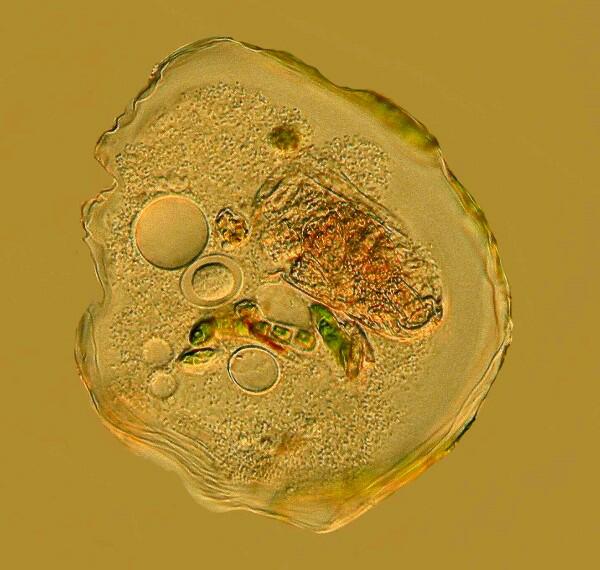
14. Thecamoeba. This specimen was taken from a dish to which I added a weak solution of the stain Neutral Red. It was sufficiently dilute that it did not kill the Thecamoeba, but it did give a vivid color to the filamentous algae which the organism then later ingested. This is a helpful way to study the feeding behavior of certain micro-organisms.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK