|
A Holiday Gallery by Richard L. Howey, Wyoming, USA |
This year I’m not going to do a rant nor present an essay, but rather, simply present 19 images with some brief comments and hope that you will find them pleasing. I have tried to select images which I think are festive and I hope that you find them so as well. Most of the people in the world have gone through a difficult year and I think in such times it is important, even crucial, to seek out small pleasures–so, I hope that you find a few bits of beauty in the images here all of which, save one, were photographed using polarized light.

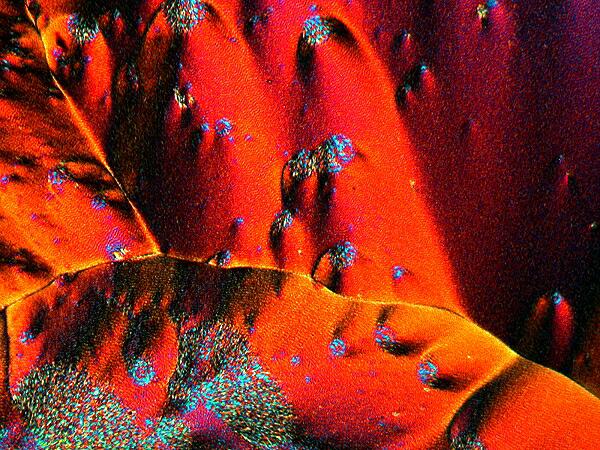
A mixture of Acetaminophen and Nickel sulfate. For some reason, this image evokes volcanic activity in my mind and I find it stark, but compelling.
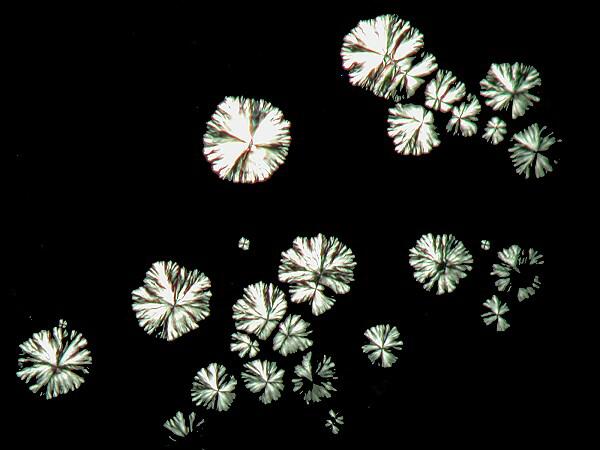
A mixture of generic Alkaseltzer and 70% isopropyl alcohol. These are clearly micro-crystalline snowflakes.
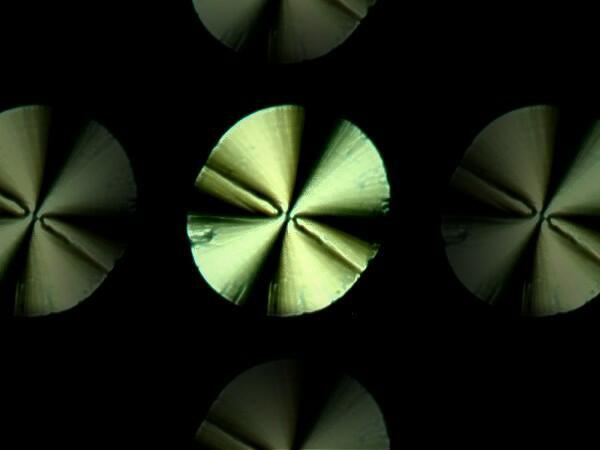
Again generic Alkaseltzer. This slide is a melt, that is, the crystals were placed on a slide and then heated until they produced this micro-disk. The reason there are 5 of them here is the result of a bit of computer graphics magic.

A mixture of Alum and Orange G. Orange G is a biological stain often used to create contrast when staining tissue. In working with crystal combinations, it is a delight as it displays an incredible variety of effects.
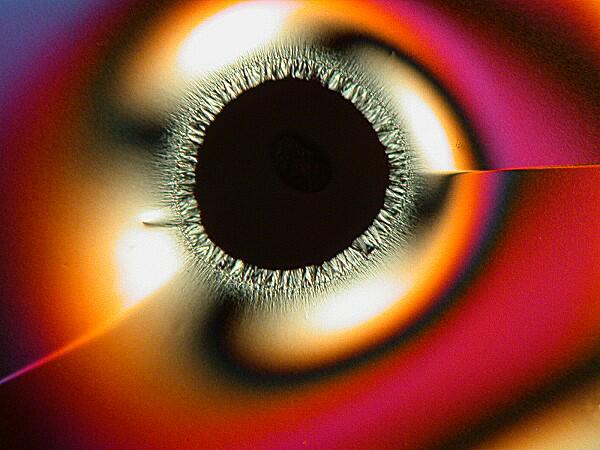
A mixture of Ascorbic acid (Vitamin C) and the biological stain Janus Green B. There is an image taken with the Hubble telescope which was dubbed the Eye of God. Well, here you have the micro version.

A mixture of Ascorbic acid and the biological stain Neutral Red. Ascorbic acid fairly frequently produces “eyes” and this image suggests to me a cat peering out from behind some foliage.

A combination of Ascorbic acid and toothache liquid. Sometimes I have used the brand name Anbesol and, at other times, the generic liquid. Both give interesting results and feathery structures like you see above are fairly common.

Sometimes a simple household substance will provide surprisingly pleasing results. This was just some Borax dissolved in water.

On the other hand, unusual combinations can produce an especially striking image such as this one of a white egret with a fragment of a sun off to the right. This was a mixture of Bromoselzer, the old-time effervescent headache and stomach distress reliever; Sodium silicate or Water Glass which was used as an egg preservative; and the biological stain Alizarin Red S.
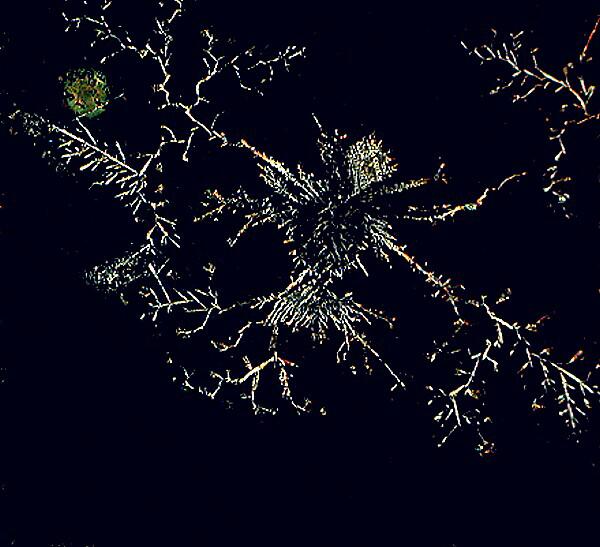
Sometimes my imagination veers off onto weird tangents and I come up with some rather strange mixes of things. For example, at one point, I asked myself what a combination of an antibiotic, Cephalexin, in this case, and some dried crystalline plant food dissolved in water might produce. Above is the result. I found the dendritic character of the image quite satisfying.

It can also be rewarding to mix a fairly common laboratory chemical, here Nickel sulfate, with a household product, in this case Efferdent which is a denture cleanser and essential if you’re old like me or if you’re young and play hockey.

Now and then, one comes across a section of a slide which displays an array of crystals which have arranged themselves in such a way as to present a cheerfully whimsical glimpse into the extraordinary micro-world of crystals. Here is a view of a slide of Magnesium chloride (used in some medicinals) and our old reliable Ascorbic acid. In this case, I look at the image and think of a kind of micro-parade of crystals as rendered by the Swiss artist Paul Klee of the Blue Rider School of Art.
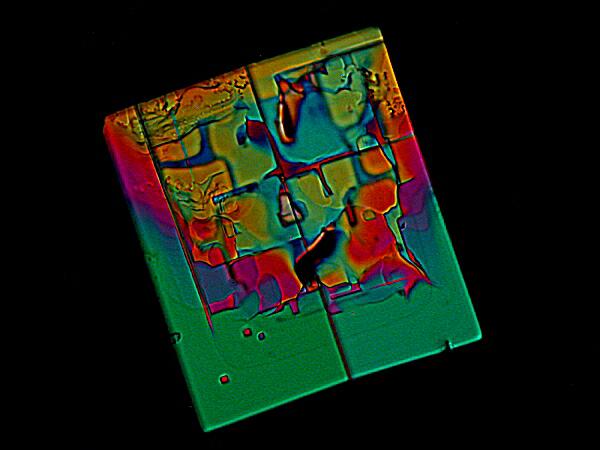
While we’re looking at Magnesium chloride, let’s consider 2 more examples. The first is Magnesium chloride mixed with the fluorescent, biological stain DAPI and what jumps out at me, again and again, is that Magnesium chloride mixtures tend to produce geometric forms.
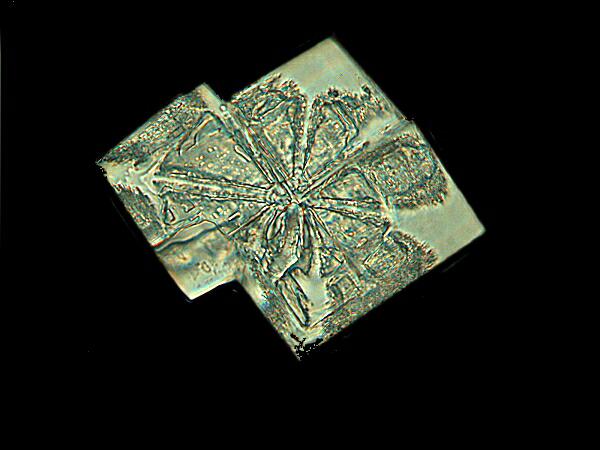
This second example, though not as colorful, is still geometric in character and consists of a mixture of Magnesium chloride and Alkaseltzer.

Let’s go back to the theme of combining a common household item with a biological stain. Here are 2 examples of such a mixture on the same slide, yet they are radically different. This was the result of a mixture of Sodium Bicarbonate (Baking Soda), Borax, and the stain Orange G.
In the first image, we see branches adorned with dendritic snowflakes.
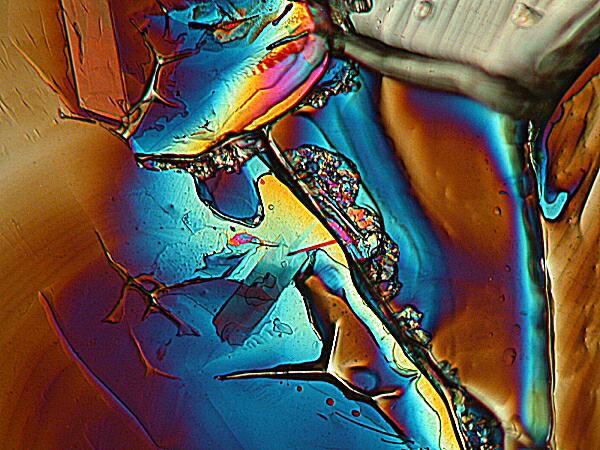
In this second image, the forms verge on the amorphous. Once again Mother Nature is throwing mud in our faces and taunting: “Hey, you think you can predict what I’m going to do?”

In this image there is a mixture of two chemicals, one of which can be extremely exasperating. This is a combination of Nickel sulfate and Rochelle salts (Potassium Sodium Tartrate)–it’s this latter one which can be annoying. Why? Because Rochelle salts are hydrophilic and pull moisture out of the air. You look at a slide and see some interesting crystals and then you go back a few hours later and they’re gone and some other crystals are there and this can go on and on for days certainly, perhaps, weeks, months, years? I don’t know; I’m not that patient. Actually, it’s rather fun, for it’s like having a magic box of tricks: now you see it, now you don’t.

Earlier we saw what the antibiotic Cephalexin produced in combination with plant food. Well, this time, I decided to see what Ascorbic acid would do when mixed with plant food and you see the result above.

Finally, this last image is a natural crystal–quartz–and is not photographed with polarization, but is a standard brightfield image but, nonetheless, it’s geometry is quite enticing.
I hope you have enjoyed this little excursion and that you will look every day for things around that bring joy and enlightenment.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the December 2010 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk .