|
Household Microscopy Part 4 by Richard L. Howey, Wyoming, USA |
Read other articles in the series.
In this essay, I want to shift the focus a bit and try to do two things: 1) consider a few substances that we havenít yet examined and 2) look at the wide variation of crystalline forms that can occur on a single slide. This will involve including a few images that you have already seen, but in the context of others which will show you the diversity I mentioned. Sometimes this can result as a consequence of taking a closeup look at a particular example on a slide or it may be a consequence of a change in the position of a compensator when using polarization. I will present these images as a sort of gallery with a minimum of comment.
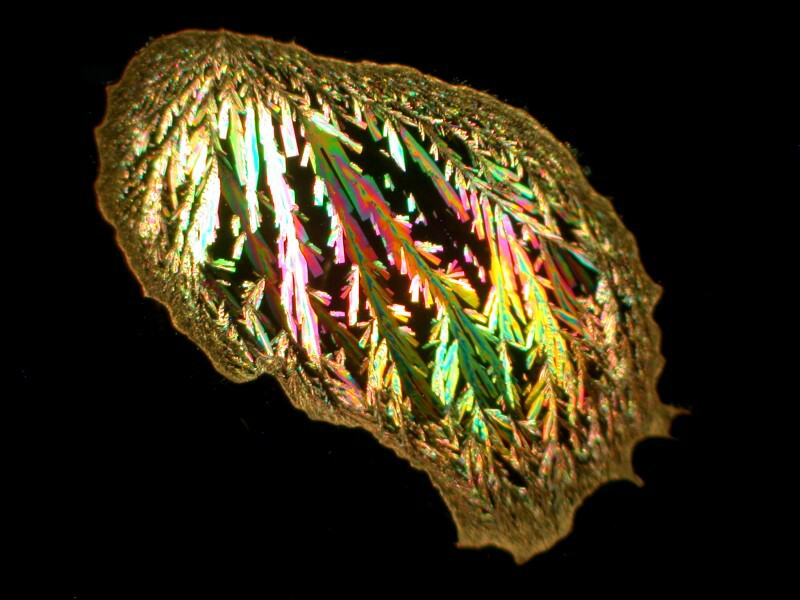
The first example is just a drop of Ascorbic acid (Vitamin C) which has crystallized in a pleasing compact form.

The second view is a closeup showing detail of some of the crystals. As you can see they are quite feathery and colorful.
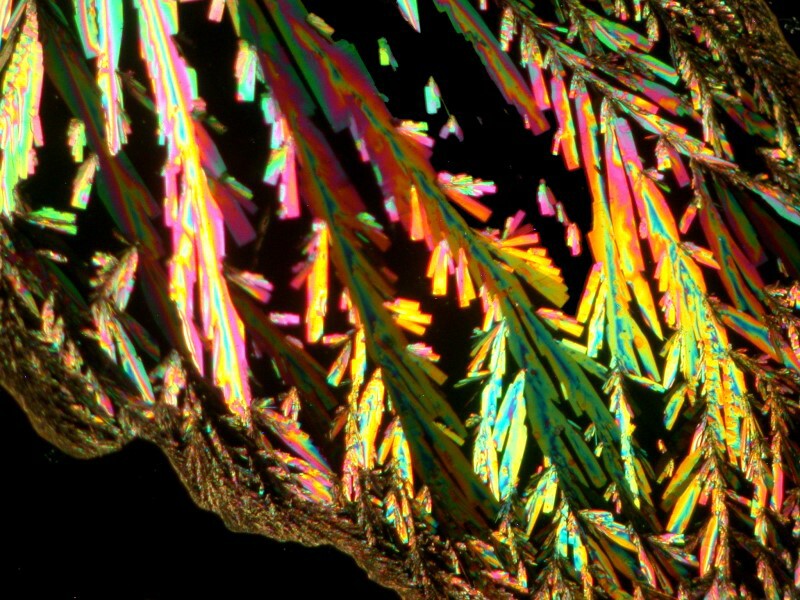
It should be noted that this is not a typical sort of pattern for Ascorbic acid; one is much more likely to get disks or areas showing a version of a Maltese cross.
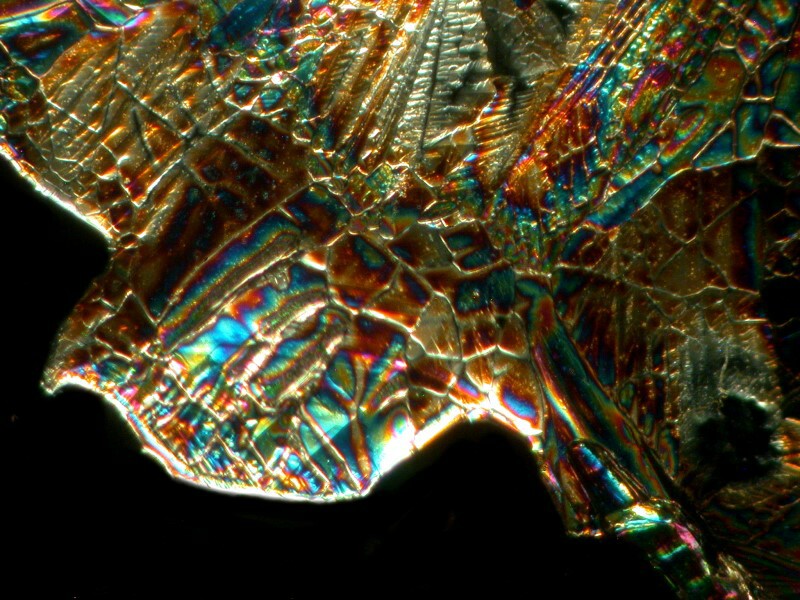
Next, letís take another common substance, Magnesium sulfate (Epsom salts). The two views I am going to show you are from the same slide and again have crystallized in a somewhat unusual fashion almost giving the impression of stained glass.

I mentioned in a previous article that Ascorbic acid is something which very often mixes with other substances to provide wonderful variations of color and form; the same is true with the biological stain Orange G which can be obtained from a supply house. It often forms acicular (needle-like) crystals in combinations. For the next 2 images, I took some Anbesol liquid (toothache medication) and did a doubling up by adding a drop of Ascorbic acid and a drop of Orange G. The results were rather like some strange cosmic birds or perhaps communication satellites.


While weíre considering Orange G, Iíll give you 3 examples of mixing it with other substances and youíll be able to see what different sorts of results one can get.
The first one is a mixture of Orange G, Ascorbic acid, and Bromoseltzer and as you can see, they combine in such a way as to create an elaborate spiny lattice.
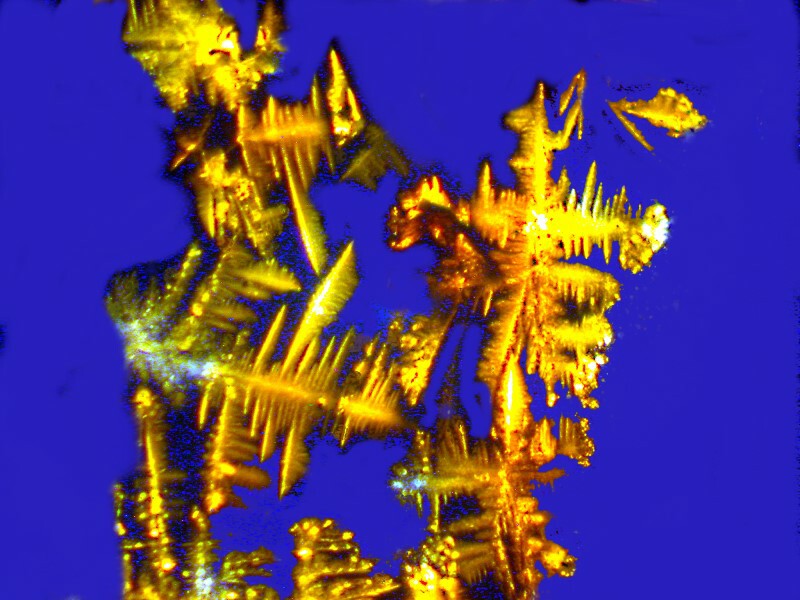
Next the Orange G and Ascorbic acid are mixed with a drop of a commercial nasal spray providing us with a very different set of forms and colors.
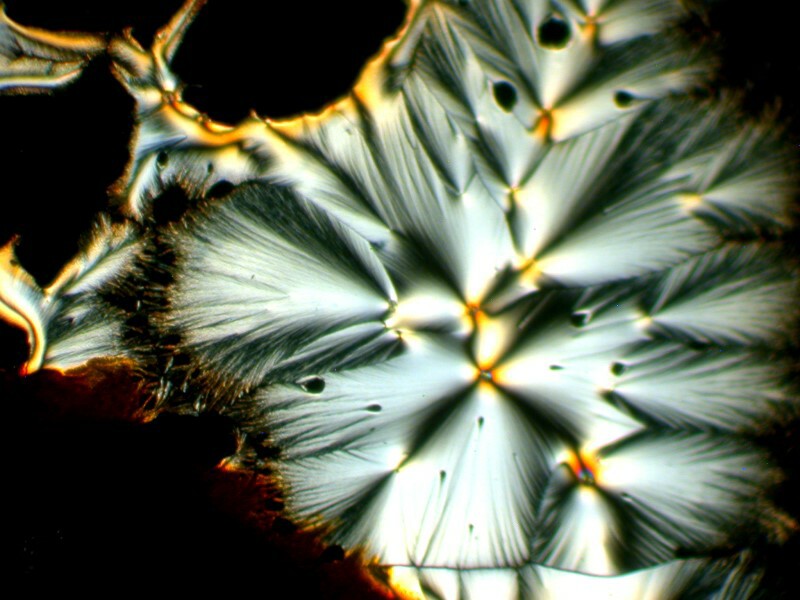
And then another very different sort of transformation by the addition of a drop of generic toothache medicine to the Orange G and Ascorbic mixture.
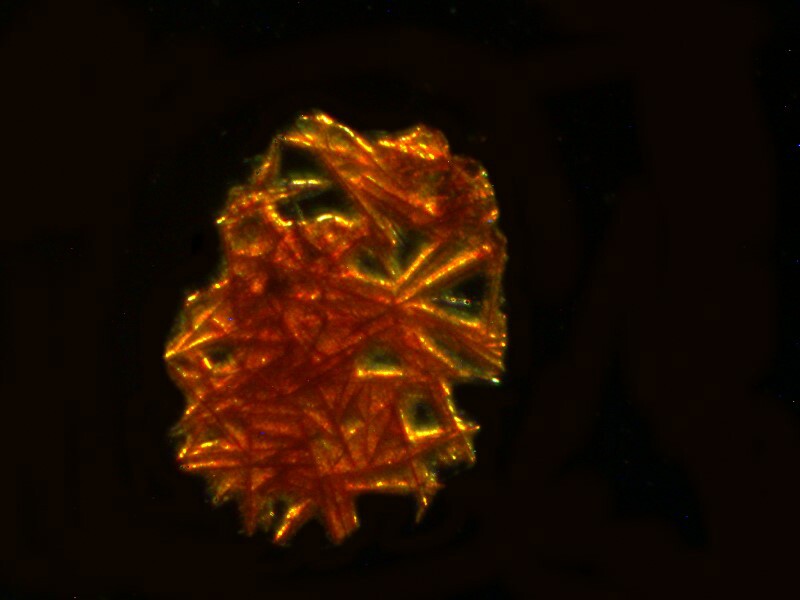
Next letís take a look at another ordinary household product mixed with Ascorbic acid, in this case, Pelikan black ink. The third image is a closeup of a section of the second, but notice how different the first image is in character and tone from the other 2.

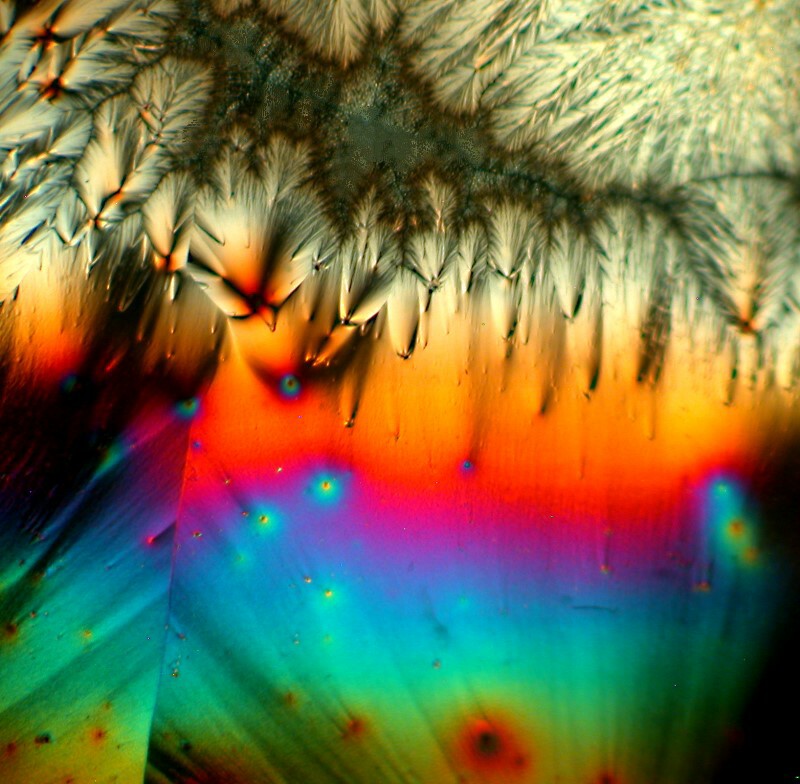
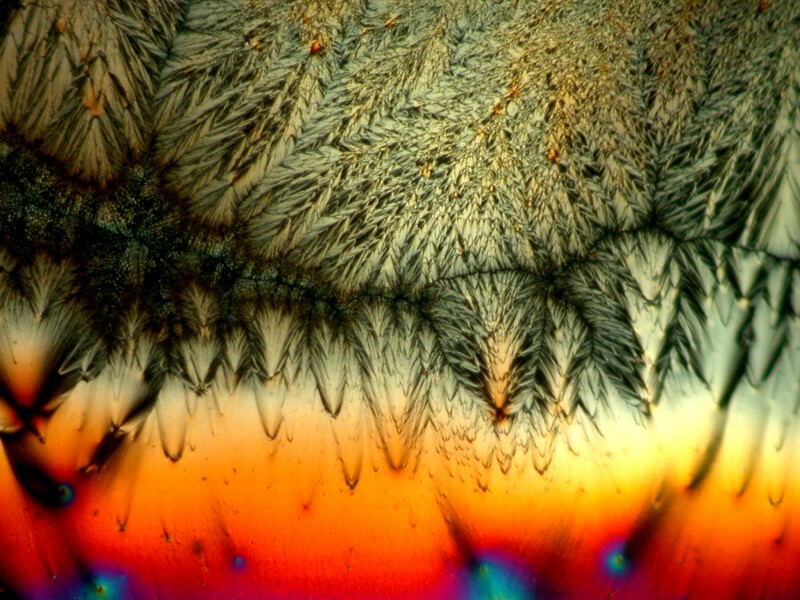
If you donít have access to biological stains, you might want to consider trying out different colored inks in various mixtures.
I was so pleased and surprised last time by the results of mixing the hand sanitizer Purell with Ascorbic acid that I made up another slide and the 4 images below are all from the same slide.
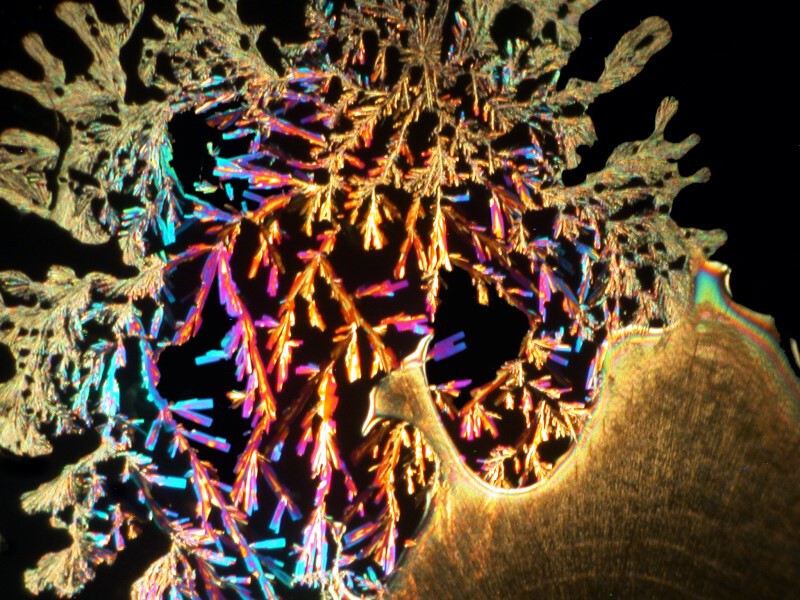
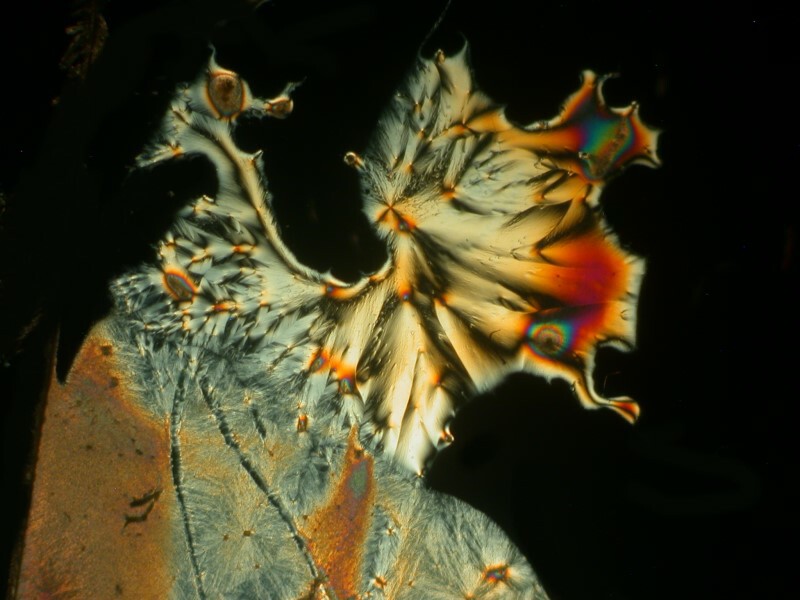
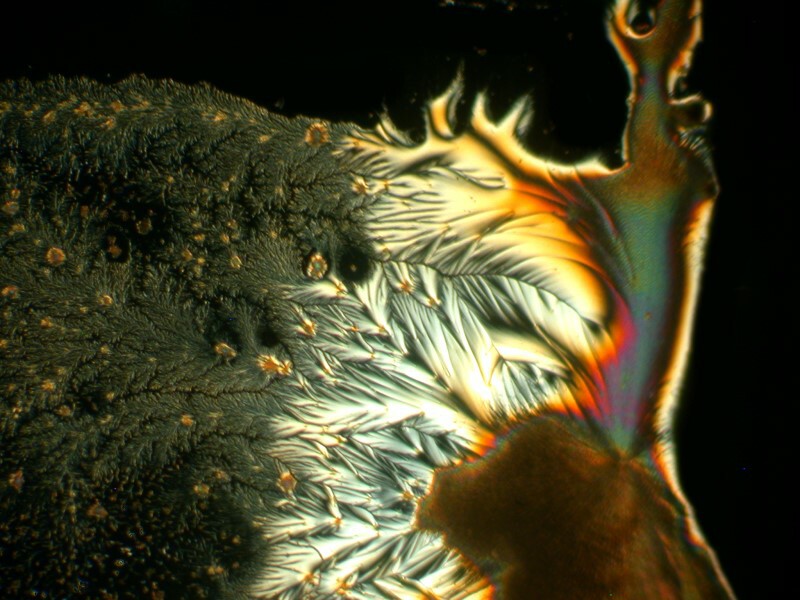
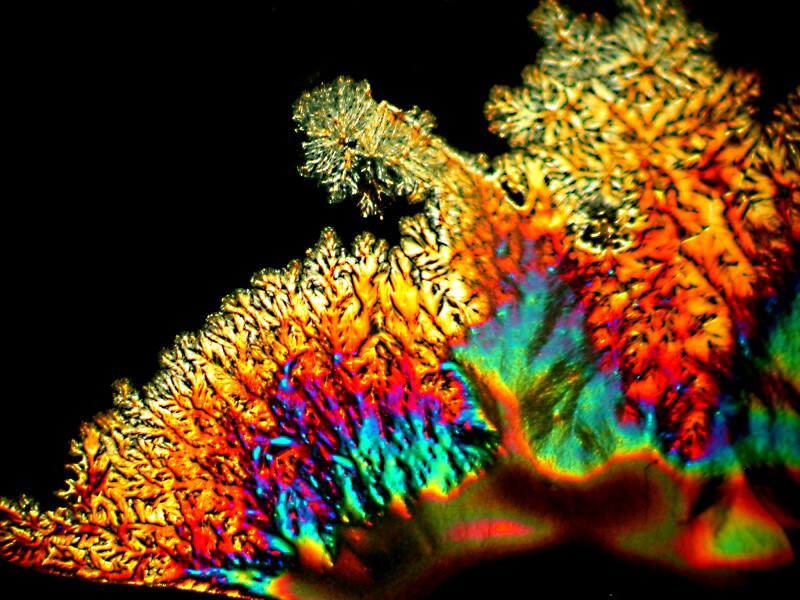
Next I tried a drop of Tannic acid with Ascorbic; Tannic acid is, as Iím sure you know, a substance found in a variety of teas.
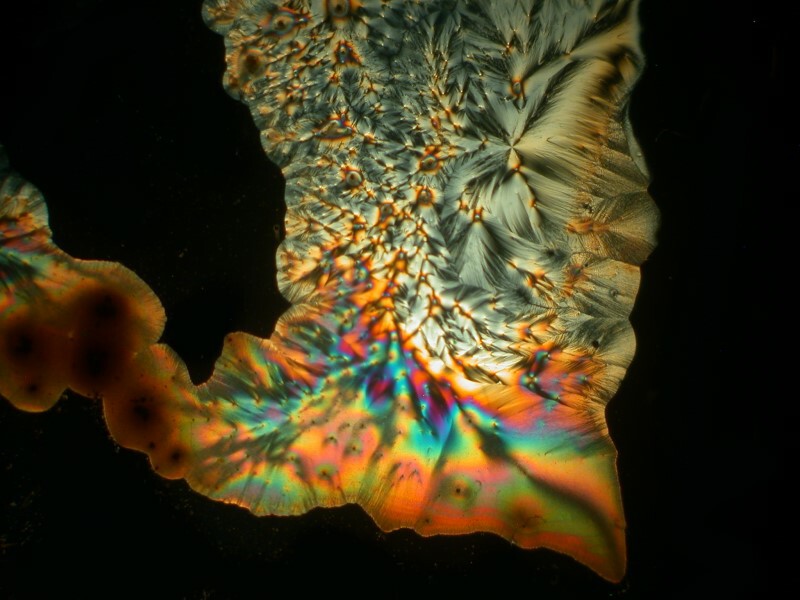
Now, we come to a compound that you may find odd to include as a household item, namely, urea. However, urea is the most common nitrogen fertilizer in the world and it is used in the manufacture of plastics and resins and can be found in some hair conditioners, facial creams, and dish soaps. Some supply houses sell the crystals in small quantities. When mixed with Ascorbic acid, it can produce some quite striking results with, at times, geometric arrangements of long acicular crystals. Iíll show you 4 examples. The last one I call ďthe door to the UnderworldĒ.

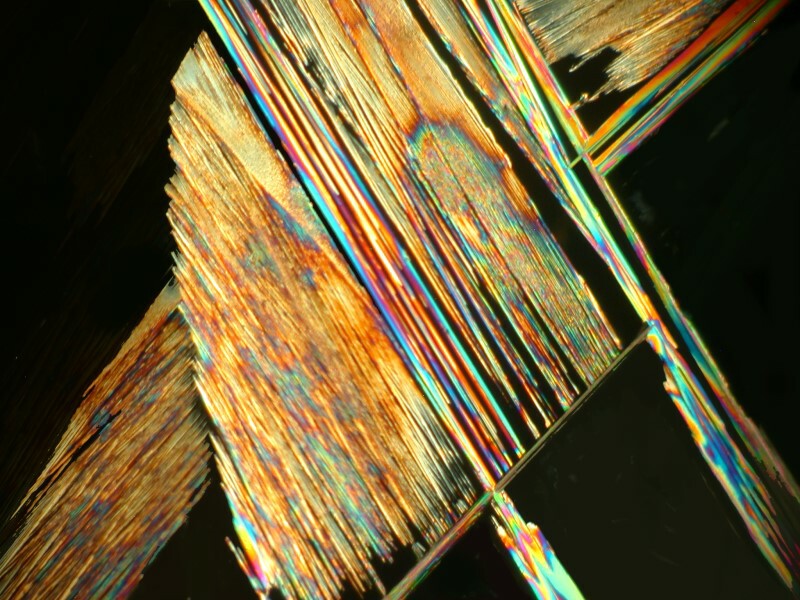
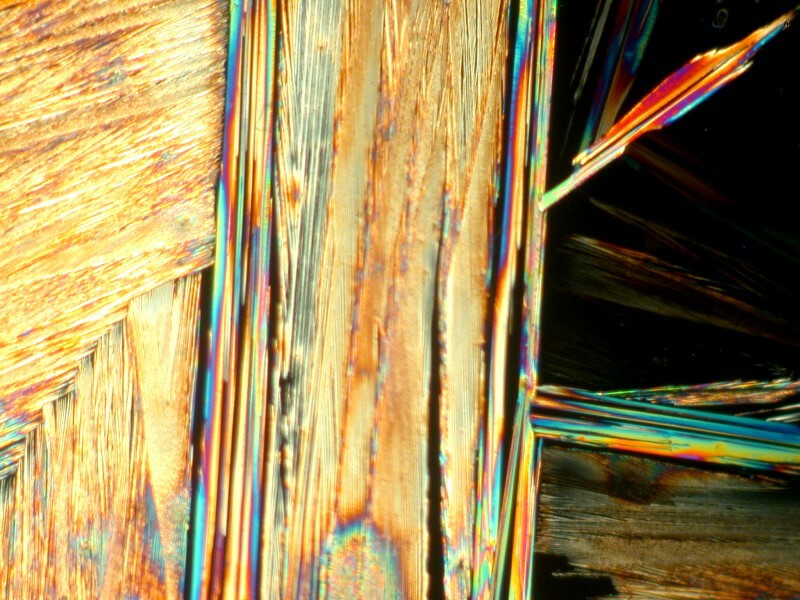
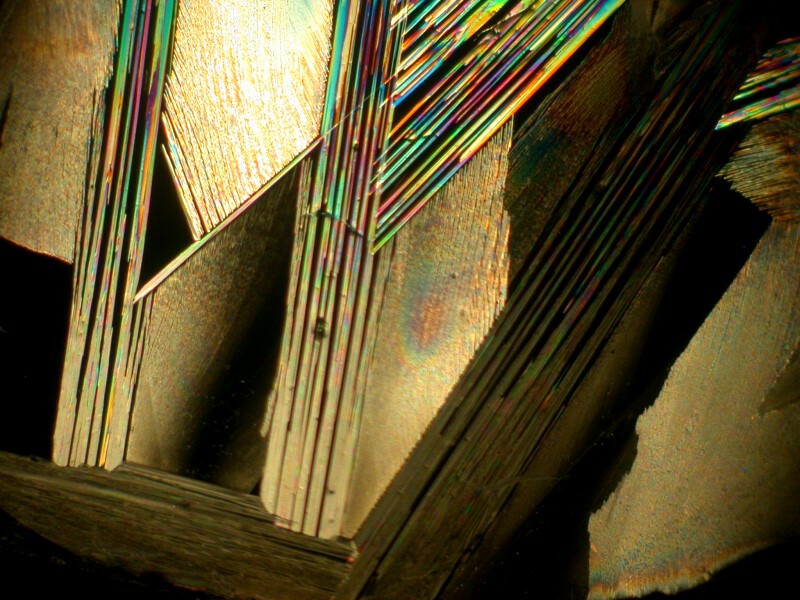
Next I decided to revisit a mixture of generic toothache medication and Ascorbic. These images remind me just a bit of some of the paintings of Joan Miro and his whimsicality.

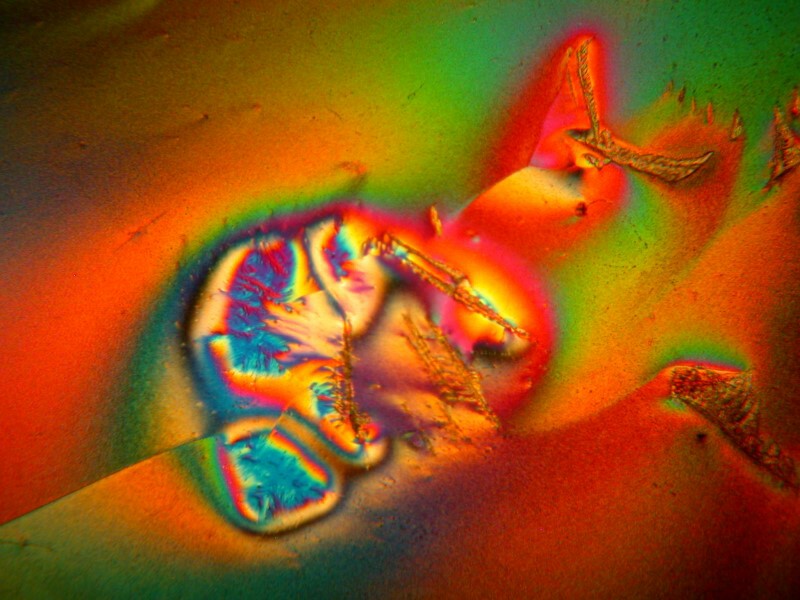
Three common substances mixed together can produce startlingly different forms on a single slide. This is a combination of Acetaminophen, Aspirin, and Caffeine.


Some Over-The-Counter medications can crystallize in unexpected ways; consider the generic NSAID Naproxen.
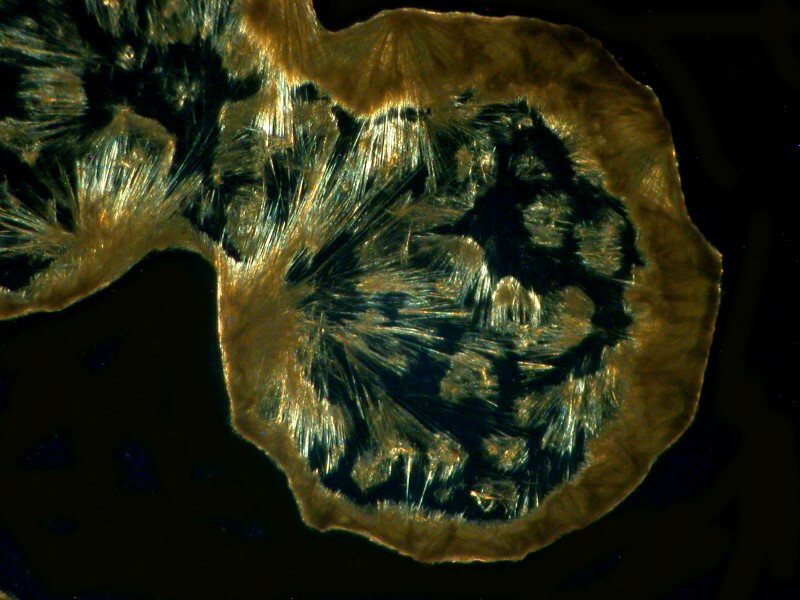
Another example is the chest decongestant Guaifenesin and the 2 images Iíll show you here are quite different in their morphology.
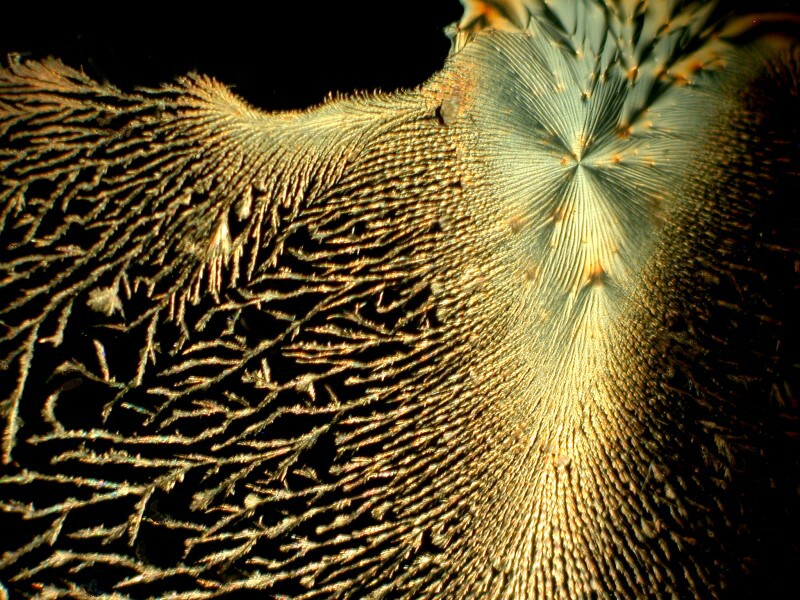

You may recall that Elmerís Gel Glue produced some interesting results that were in the form of large droplets. Here Iíll show you one that is and one that isnít.
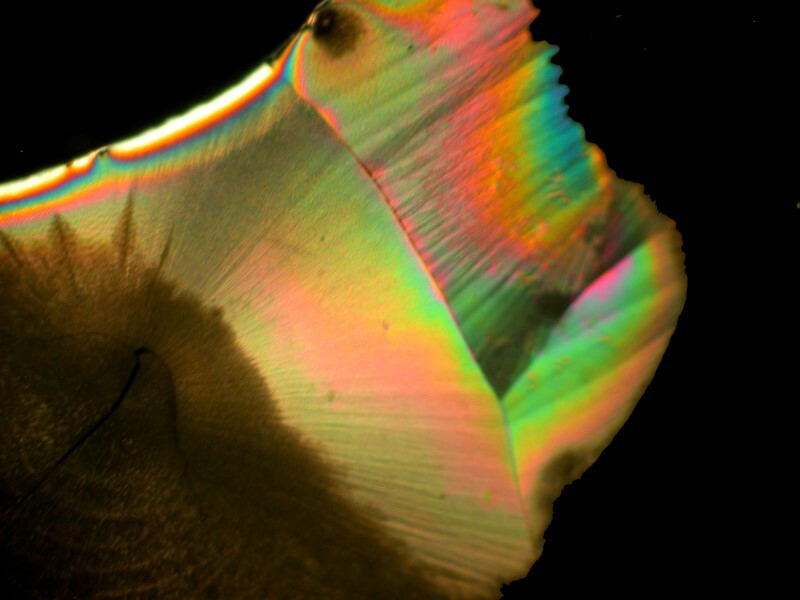

Valerian is from a plant and is incorporated into a variety of stress-relieving and sleeping aids. A bit of it mixed with Ascorbic acid produced an amoeboid-like form with 2 smaller internal structures which could almost be seen as nuclei.
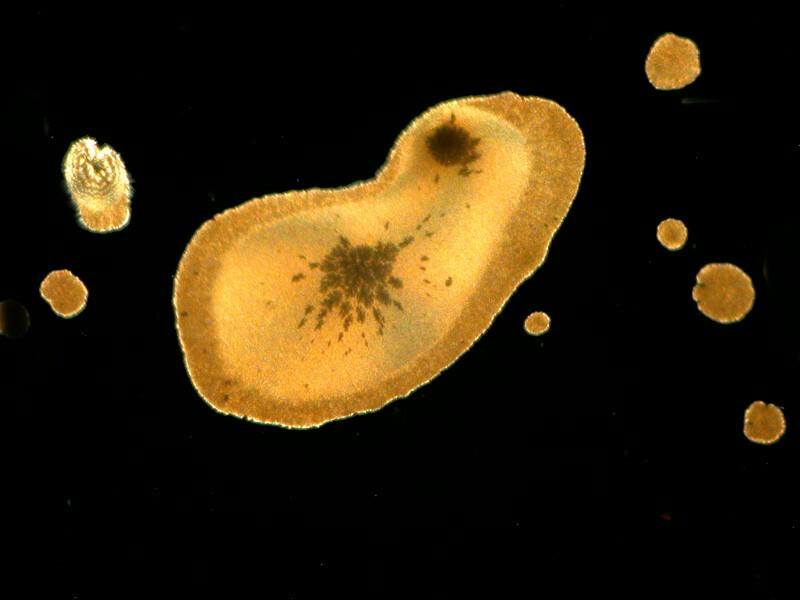
Simethicone which is used to relieve intestinal gas, when mixed with Ascorbic, can present itself in very different shapes. In the first image we have some wonderful disk shapes and in the second a form that could almost be a cosmic butterfly.

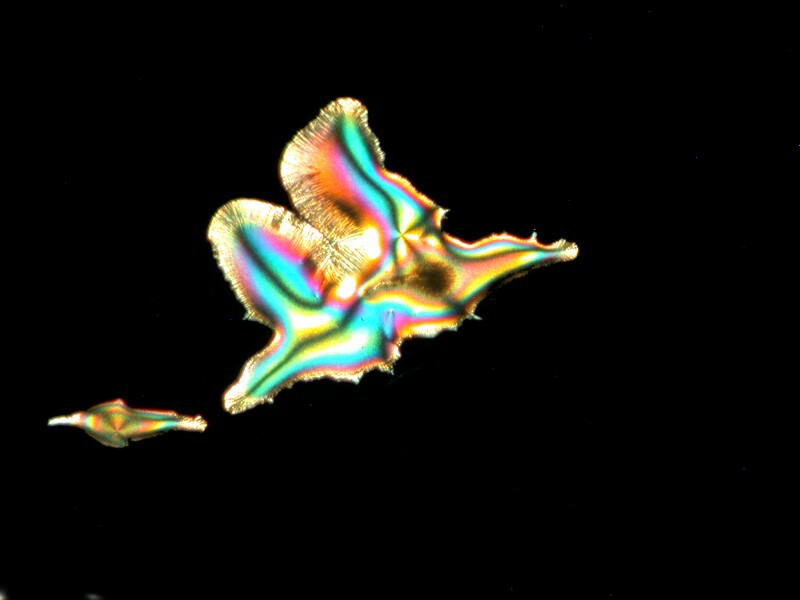
You may recall that the major ingredient in Purell is isopropyl alcohol. Well, alcohol added to a mixture can sometimes give us remarkable diverse images. The Elmerís Gel Glue that we looked at above when treated with Ascorbic and a drop of isopropyl provided 3 very different images from the same slide. The first could perhaps be described as a crystalline super-nova, the second as a feather fan, and the third as an aerial view of a volcanic island.
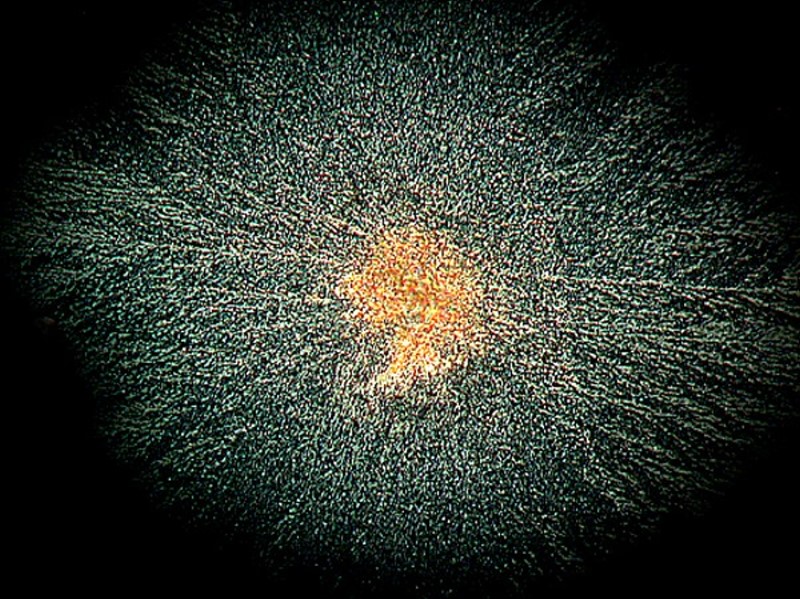

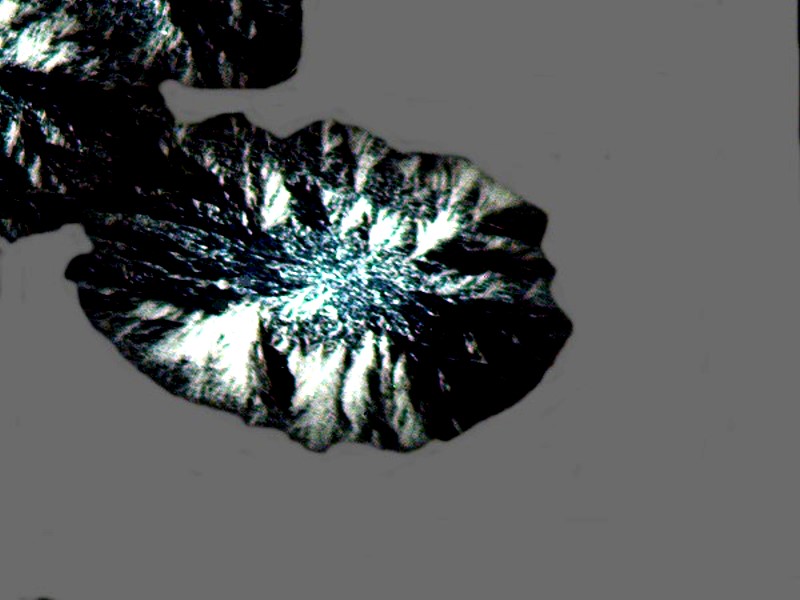
Another kind of medication for relieving the pain of toothache, canker sores, or denture irritation of the gums is called Kanka. Mixed with a bit of Ascorbic it produced a fine image of what could be a Native American feathered battle shield.
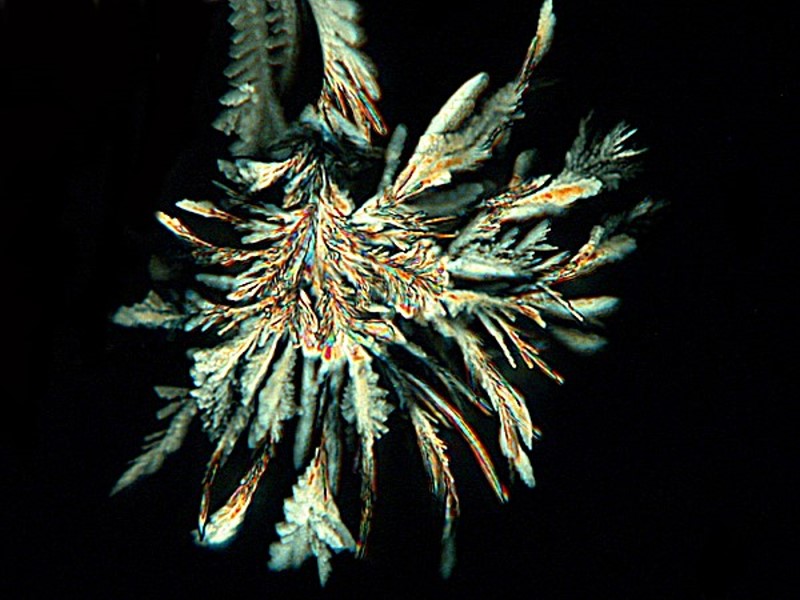
Above we looked at some examples resulting from adding a bit of isopropyl alcohol. When the alcohol and the water come together we get a swirling turbulence that often mixes things in interesting ways. Below are two examples of Ascorbic treated with alcohol. Here the stratification is particularly interesting to me.
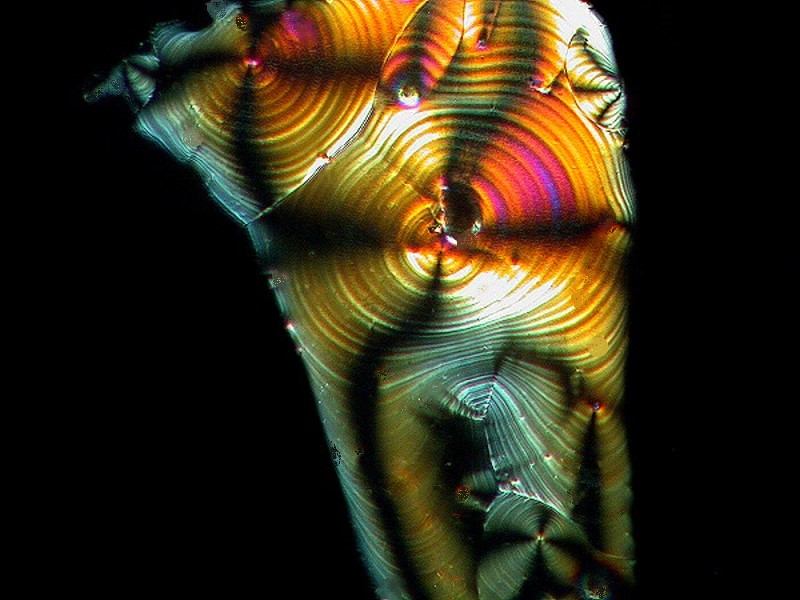
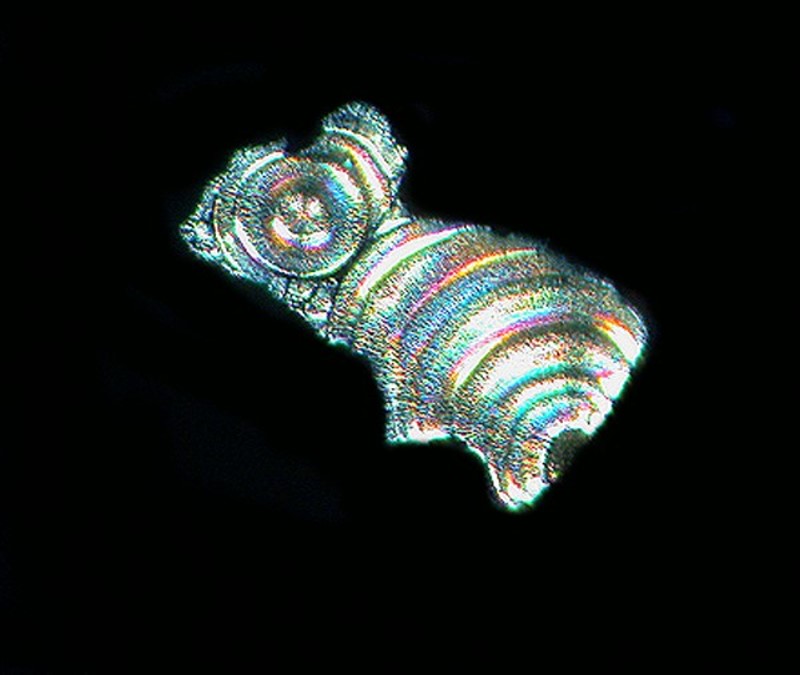
Another common household product, Borax, when mixed with Ascorbic, produced the image below.

There are so many possibilities and in my medicine chest I found a small bottle of callus remover which smells like it should indeed produce something intriguing. The 2 images show very different forms on different parts of the slide.
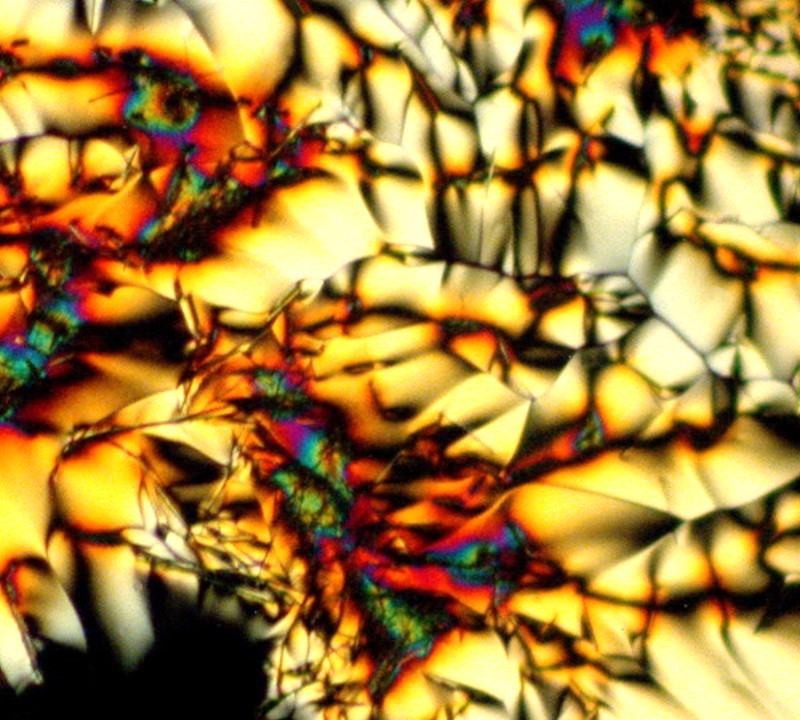
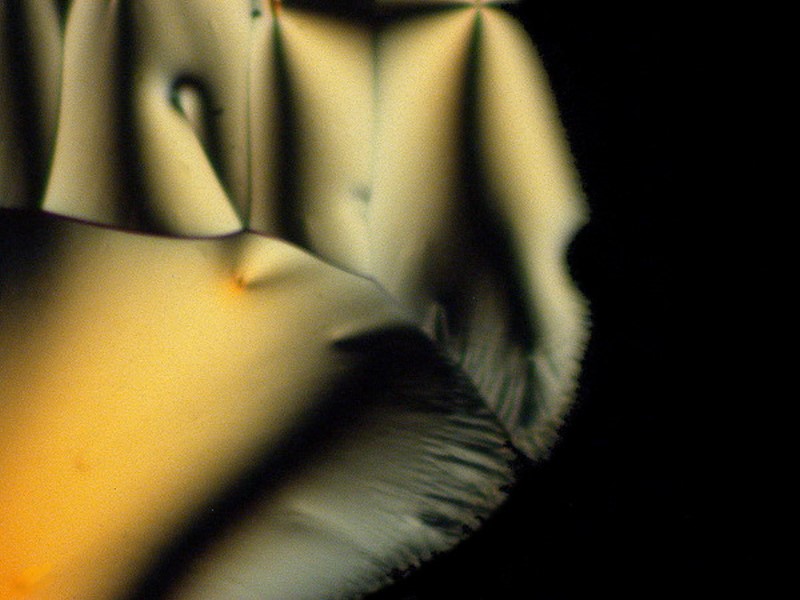
The next 5 images illustrate especially well my notion that the same substance can produce striking variations such that, unless one knew in advance that they all were from the same slide, one would very likely not think that they were. These are a mixture of tincture of iodine and Ascorbic and because it is a tincture, we again have alcohol playing a role in producing these crystals.




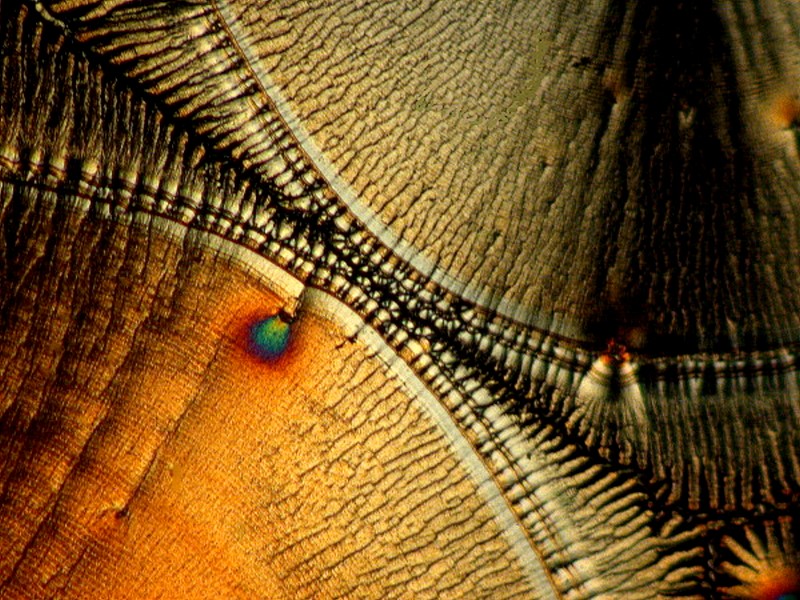
Finally, I will conclude with 3 images that once again involve introducing a bit of material from an organism; in this case, a tiny fragment of calcareous material from the spine of a sea urchin. Here we have lovely disks and Maltese crosses that are typical signatures of Ascorbic acid.
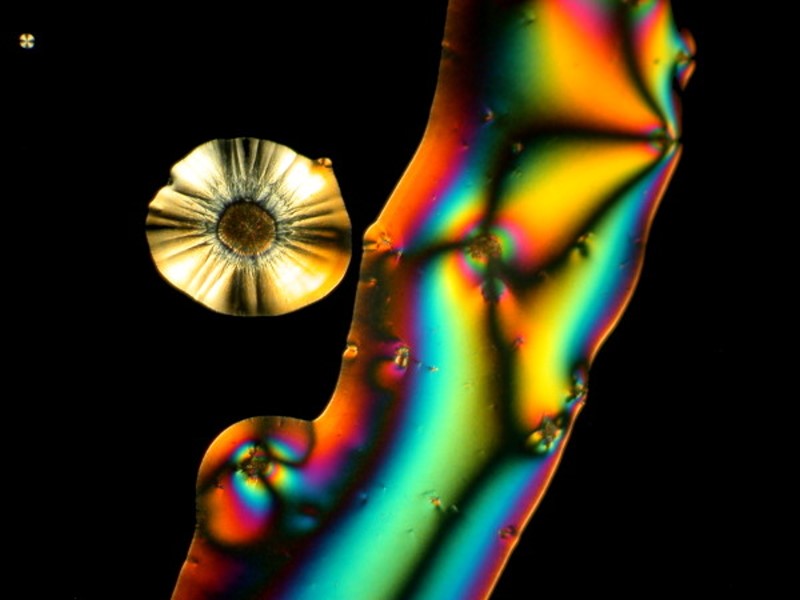
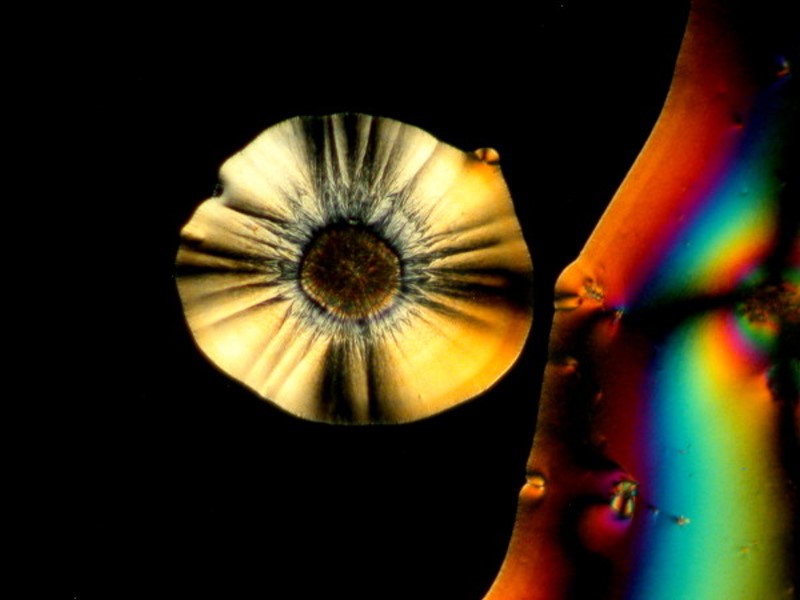
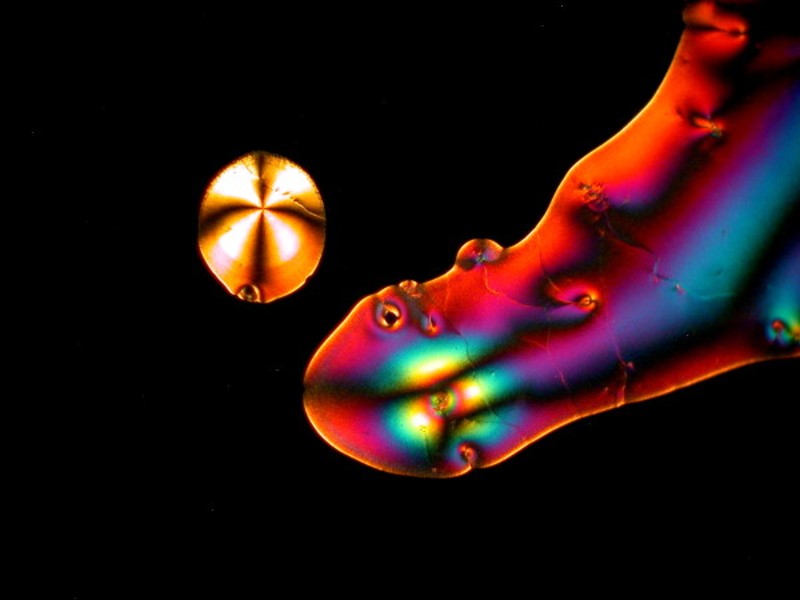
I hope you enjoyed this gallery and in Part 5, weíll look at some different sorts of material which will most likely be other than crystalline.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the February 2016 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .