|
|
A Gallery of Caffeine Photomicrographs
( using a variety of illumination techniques ) by Brian Johnston (Canada) |
|
|
A Gallery of Caffeine Photomicrographs
( using a variety of illumination techniques ) by Brian Johnston (Canada) |
Caffeine is a central nervous system (CNS) stimulant, and is considered by many to be the world's most popular drug. Coffee, tea, cocoa and chocolate products contain the substance, and therefore all tend to increase mental alertness. Soft drinks as well usually contain caffeine.
In the medical arena, it is sometimes used in the formulation of headache medicine and in pain relievers where it helps analgesics work more efficiently.
Caffeine is a member of the group called xanthines. As the structural formula and molecular shape show, it is a complex molecule composed of two rings containing carbon and nitrogen atoms. (The illustrations were produced using HyperChem.)
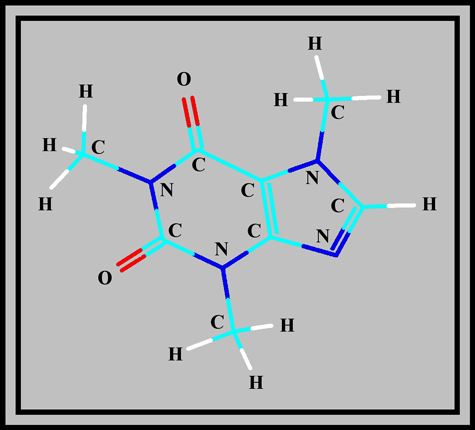
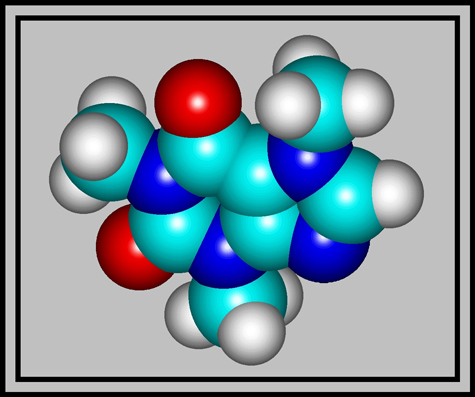
White crystals of the compound have a melting temperature of about 238 degrees Celsius, but sublimation begins at 178 degrees Celsius. Although the melting point is rather high, it is possible to prepare a melt specimen by heating a few crystals sandwiched between slide and coverglass with an alcohol lamp.
Although this compound is ubiquitous, it is normally at very low concentrations in the products that we ingest. It is however, very toxic in the pure form! (As little as 10g will usually be lethal in a human.) The MSDS safety data states: “Warning! Harmful if swallowed or inhaled. Causes irritation to skin, eyes and respiratory tract. Possible birth defect hazard. May cause birth defects based on animal data.”
The images in the article were photographed using a Nikon Coolpix 4500 camera attached to a Leitz SM-Pol polarizing microscope. Images were produced using several illumination techniques: dark-ground, phase contrast and polarized light. Crossed polars were used in all polarized light images. Compensators, (lambda and lambda/4 plates), were utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x or 25x flat-field objective formed the original image and a 10x Periplan eyepiece projected the image to the camera lens.
With polarized light (crossed polars), and low magnification, caffeine fields are often composed of large colourful areas with random shapes. It is difficult to see that each area has very fine cracks or fault lines which tend, for the most part, to be parallel. (Look particularly at the gray area on the left side.)
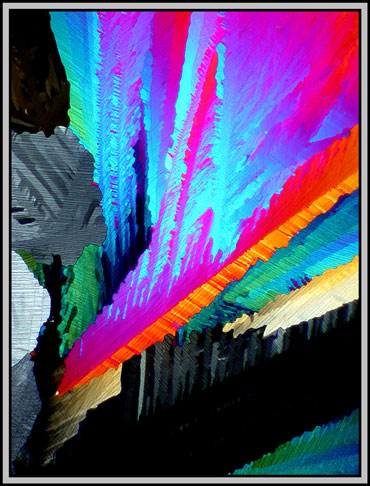
With a slightly higher magnification the fault lines become clearer. The two images below are of the same field. In the image to the right, the microscope stage and slide have been rotated slightly in a counterclockwise direction. Notice how some of the colours have changed as a result of this rotation.


Colour change can also be produced by using compensators that alter the phase of the light rays passing through the crystals. Additional control is possible because one of the compensators can be rotated. Once a particular crystal field has been chosen, it is therefore possible to obtain almost any colour pallet. (Of the three images below, the first, on the left is my favourite.)



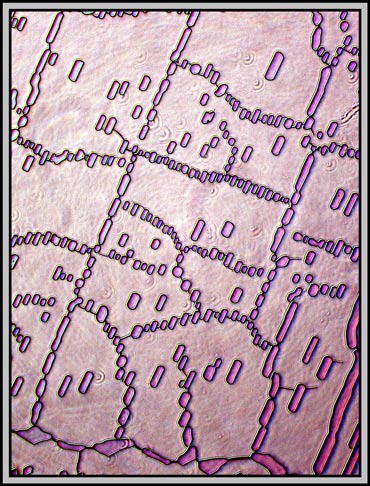
At higher magnification, the cracks or faults become so distinct that they can interfere with the attractiveness of the image. This is the case in the yellow-orange area of the photomicrograph below. The fault lines clearly are not parallel, and this proves an unpleasant distraction.
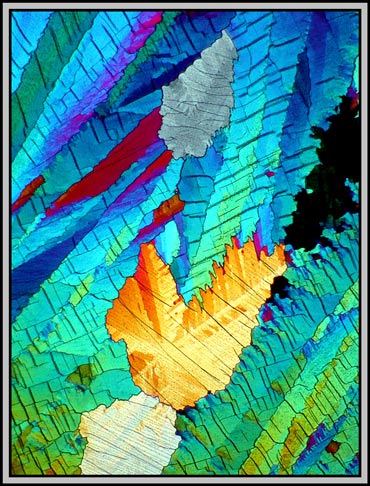
If, as the melt recrystallizes, some slight pressure is applied to one portion of the coverglass, the resulting crystal layer will be thinner. Under crossed polars, this often results in images in shades of gray. It is in areas like these that compensators can add colour and produce quite attractive results. The differences in the two images below are caused by a very small rotation of one of the compensators. Note that many of the fine lines in the low magnification photomicrographs are actually trails of microscopic bubbles! (This is probably due to the low sublimation temperature.)
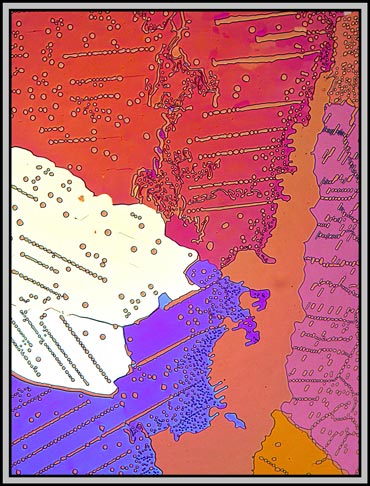
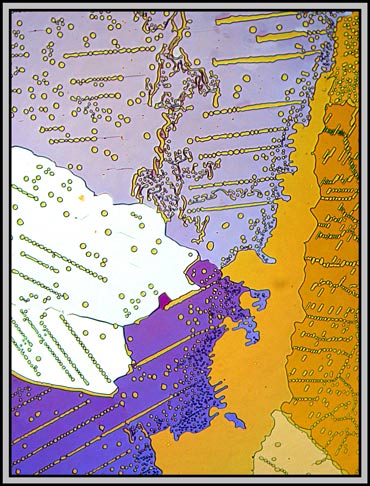
Again, it is compensator rotation that alters the colours in the following two images.
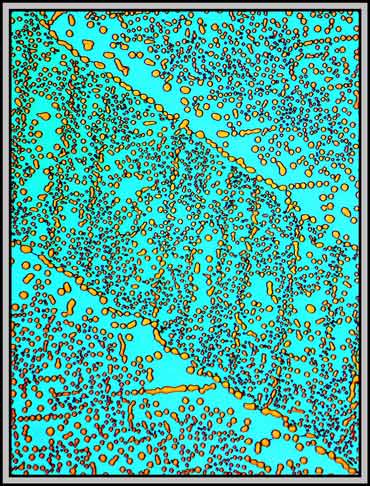
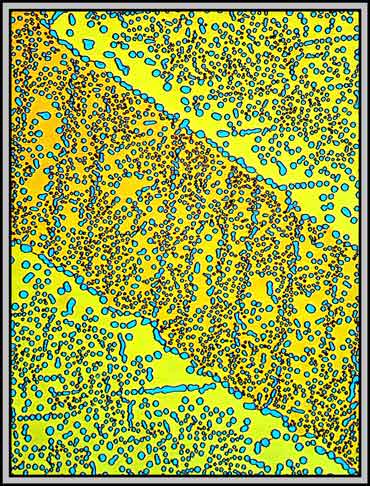
The image below is interesting because it shows two different types of bubbles. The first type, that exists in both areas, is comprised of randomly positioned bubbles. The second has the bubbles positioned right over a fault line. (I have no idea why the two parallel diagonal lines in the image have no bubbles associated with them!)
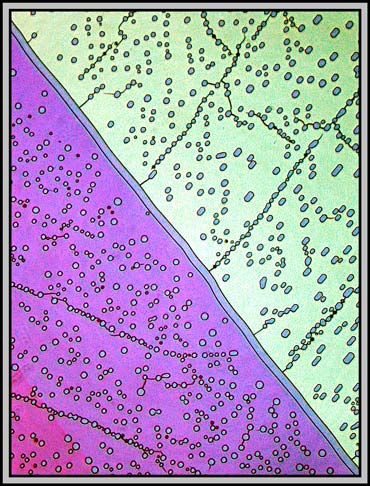
Even more interesting is the fact that not all of the bubbles are round! The images below, taken at higher magnification, show rectangular bubbles with rounded ends and, in the right hand case, irregular shapes. These photomicrographs were taken using a phase contrast condenser with one of the phase rings in position, and a normal, (not phase) objective.

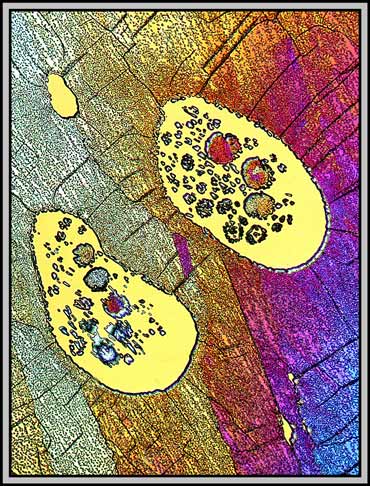
Earlier in the article it was mentioned that caffeine begins to sublime (solid to gas) long before it melts. As the melt solidifies, this gaseous caffeine may then sublime again (gas to solid) into any large voids existing between coverglass and slide. The resulting blobs are striking in the polarized light image below.
Using a proper phase contrast arrangement (condenser and phase objective), provides a different view of the structures in the melt specimen. The first image is of an area near the edge of the coverglass, while the remainder cover central locations.
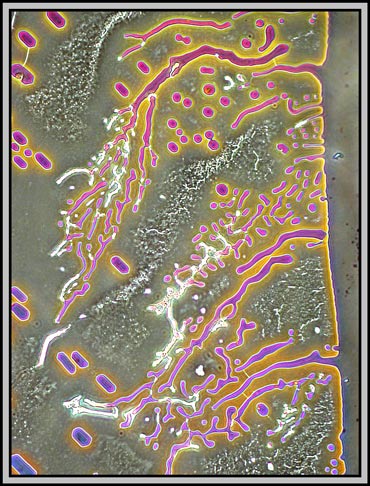
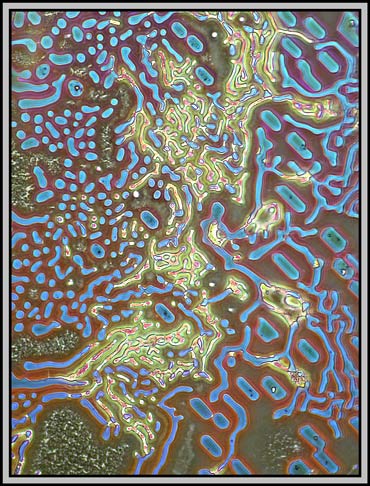
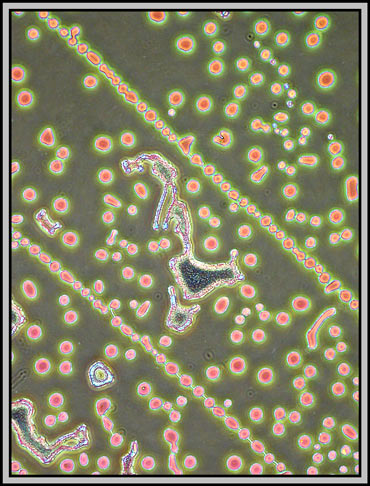
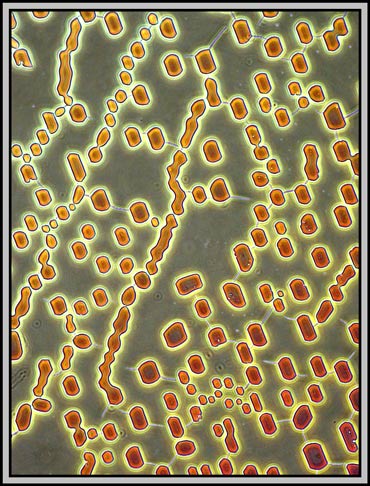
A dark-ground condenser reveals that the slide is an absolute maze of fine faults.

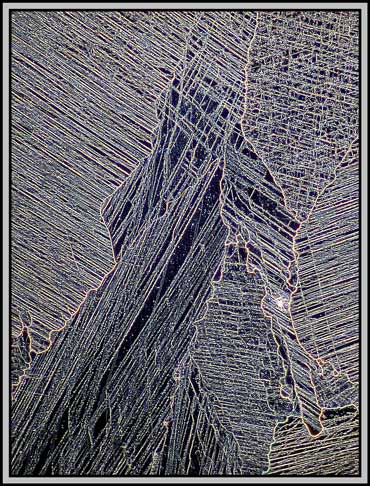
The remaining images were taken using one of the phase rings of the phase-contrast condenser and a non phase objective. The result is essentially dark-ground in nature, but with colours produced by interference phenomena. (Sometimes it pays not to follow the rules!)

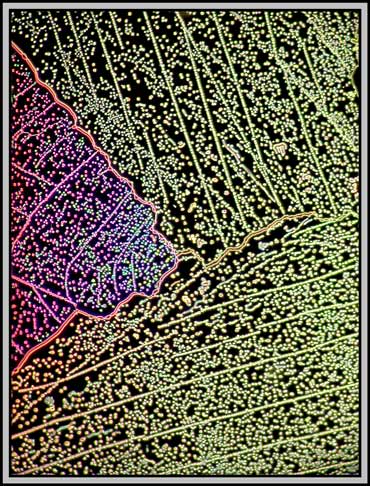


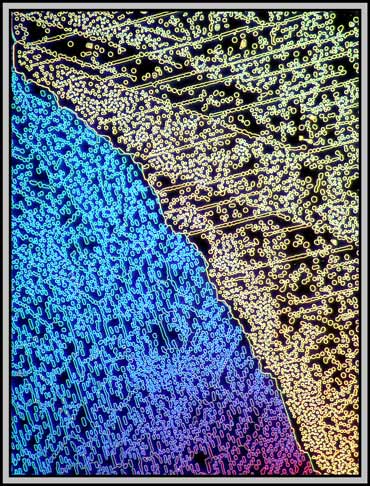
Twentieth century microscopes are certainly technological masterpieces. It takes only seconds to alter the illumination method, and thus provide a completely different perspective on the subject being studied. This technological advance has provided an array of even newer visualization techniques over the last few years, but unfortunately, the cost has risen as well, putting the equipment out of the reach of the ordinary person!
All comments to the author Brian Johnston are welcomed.
Published in the January 2005
edition of Micscape.
Please report any
Web problems or offer general comments to the
Micscape
Editor.
Micscape is the on-line
monthly magazine of the Microscopy UK web
site at
Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .