|
|
A Gallery of n-Butyl-p-Aminobenzoate Photomicrographs (using a variety of illumination
techniques) |
|
|
A Gallery of n-Butyl-p-Aminobenzoate Photomicrographs (using a variety of illumination
techniques) |
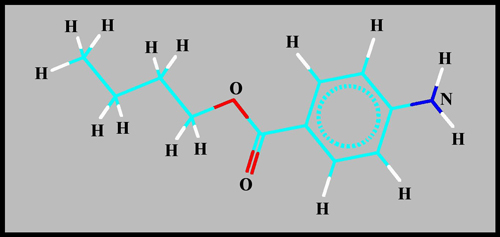
N-butyl-p-aminobenzoate
is used as an intermediate in the manufacture of other organic
compounds. The substance itself is used in some suntan lotions to
block sunburn producing ultra-violet radiation. It has as well,
some small application as an anesthetic in medicine.
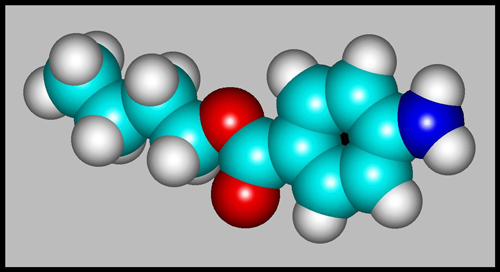
The structural formula and
molecular shape can be seen below. (HyperChem was used to produce
both illustrations.)
Since the pale yellow-white
crystals have an exceptionally low melting temperature, 58 degrees
Celsius, it is easy to produce a melt specimen by gently heating
a few crystals sandwiched between microscope slide and
coverglass. Note however that the MSDS safety information about
the compound warns that a fume hood should be used, and that skin
contact with the substance be avoided.
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using several
illumination techniques: dark-ground, phase contrast and polarized
light. Crossed polars were used in all polarized light
images. Compensators, ( lambda and lambda/4 plates ), were
utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x
or 25x flat-field objective formed the original image and a 10x
Periplan eyepiece projected the image to the camera lens.
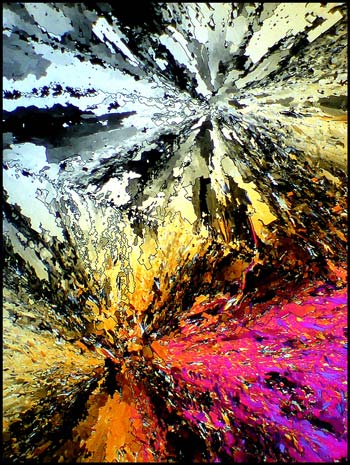
The first image in the article
shows a typical field at low magnification. It uses crossed
polars, and two lambda/4 compensators, one above and one below the
slide, to alter the colour. Exactly the same field without the
use of compensators can be seen below left. To the right is a
higher magnification image of the centre of the upper star-burst shape.

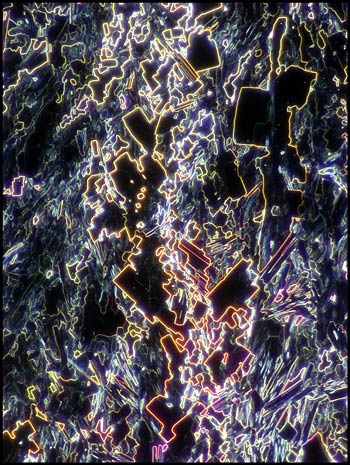
Many of the fields on the typical
slide contain complex right-angled shapes. These forms can be
clearly seen in the dark-ground image below.
The compensators mentioned above
allow almost limitless control over the colouration of the final
image. To the left below is an image formed by crossed polars
that uses two lambda/4 compensators. Since one can rotate,
additional colour control is possible. Compare this image with
the one to the right, and the first image in the article. A small
rotational adjustment in each case is the only difference.


The colour intensity of any
particular location on the slide depends on the thickness of the
crystal layer between the slide and coverglass. If the layer is
too thick or too thin, the colours appear muted. The trick is to
obtain the ‘perfect’ thickness. Unfortunately, trial and error
seems to be the only way to the ‘ideal’ image. (It’s for this
reason that about three out of every five melt specimen attempts are
useless garbage! The problem is that, unlike other garbage, this
contains potentially hazardous chemical material and cannot simply be
thrown out in the trash. Since there is no inexpensive way of
disposing of old slides, I keep a large screw-capped jar in which all
of my failures over the last thirty years reside!)
After a successful slide has been
prepared, one must choose an area to photograph, and a magnification
that will reveal the detail that is important. The image below
shows a medium magnification photomicrograph of the radial arms of a
star-burst. The apparent structure of the image is produced by
the red colour of the arms
contrasted against the blue background.

By contrast, it is the crystal
shape in the three images below
that determine the apparent structure
in the image. Notice that although compensators have been used to
alter the colour dramatically, the viewer easily discerns that the
shapes have remained constant.


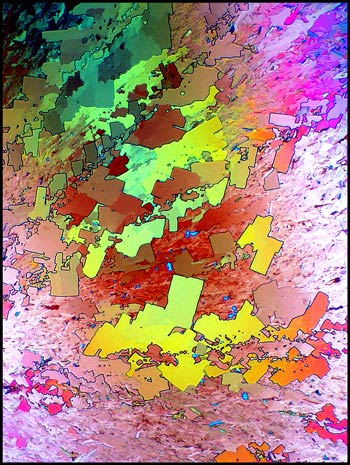
All of the images in the article so
far have been from the central area of melt specimens. For the
remainder of the article, it is at the edges of the sample, near
the coverglass margins, that the images have been obtained. As an
experiment, I ringed the edge of each coverglass with a bead of nail
polish. This action turned out to be serendipitous, for the
solvent in the nail polish acted as a solvent for
n-butyl-p-aminobenzoate. The melt crystals near the coverglass
edge first dissolved, and then recrystallized to form fascinating new
fields of view.
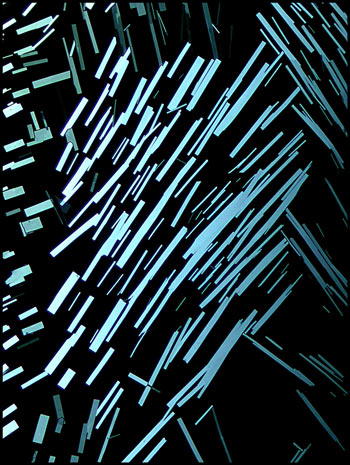
Some of the crystals that
recrystallized from the solvent had the perfectly rectangular shape
that can be seen in the left (dark-ground) and right (crossed polars)
images.

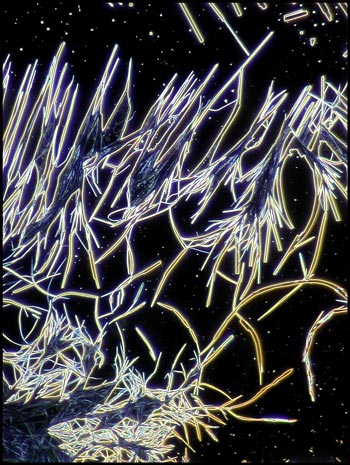
Other locations displayed branching
feather-like structures as well. (Both images use crossed polars
with both lambda/4 and lambda compensators.)


Under dark-ground illumination, the
edges of the feather-like structures stand out clearly.
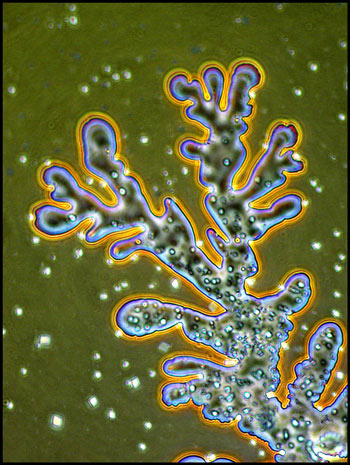
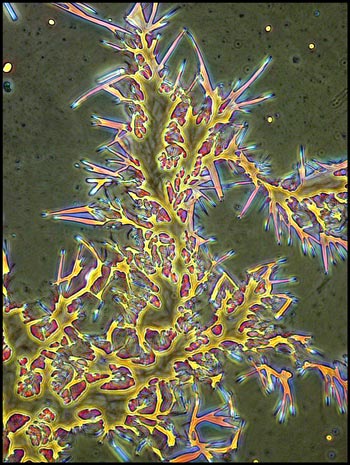
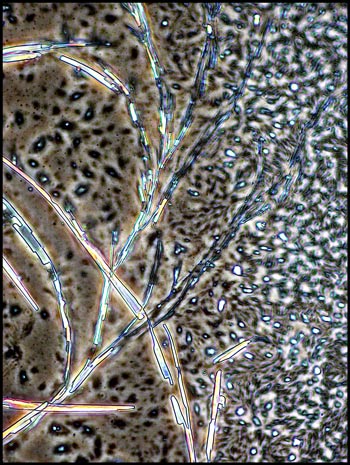
At the very edge of the coverglass,
some very unusual structures formed that only phase-contrast
illumination reveals clearly.

I remember that the study of
optical crystallography at university was an onerous task, with complex
math and terminology. It is certainly more fun to simply wonder
at the beauty produced when molecules attract to form the regular three
dimensional lattices that we call crystals!
Published in the
January
2006 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1996 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .