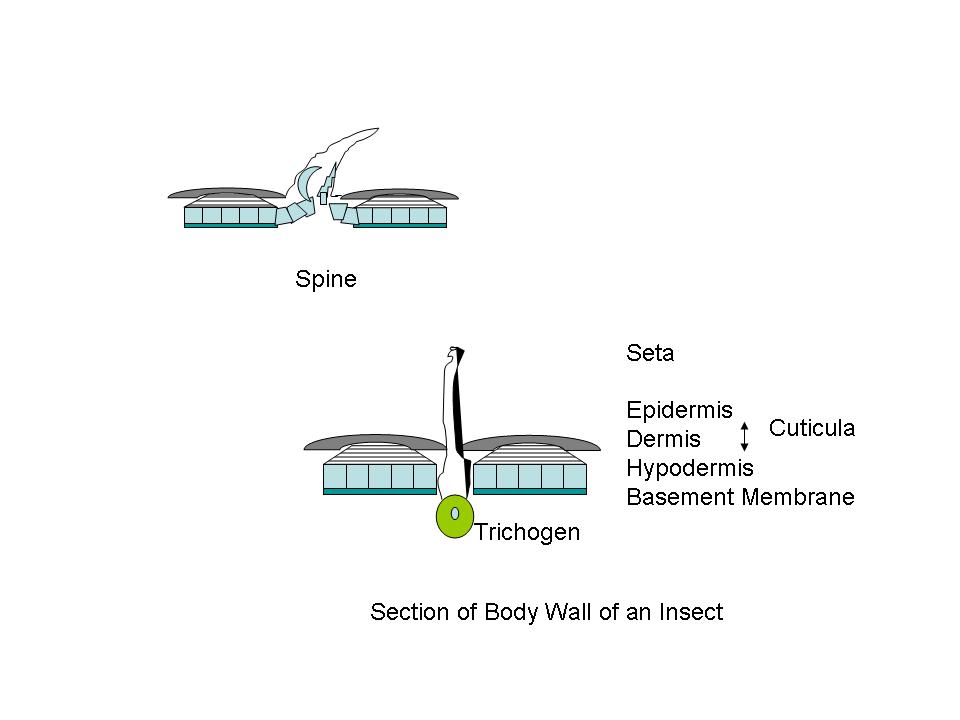
Figure A Body wall, diagrammatic cross sectional structures;
Figures adapted from Introduction to Entomology, J. Comstock, 9th Ed., 1940
'Hairy' Insects and Spiders
Spurs, Spines, Setae, and Sensilla.
by Ray Dessy, Blacksburg, VA USA
ABSTRACT: As mammals with mammalian friends, we are familiar with hair, fur, eye-lashes, beards, coats, manes and tails. We seldom recognize the similar projections that some arthropods possess, unless we look carefully. This article illustrates some of these analogs, and discusses their functions. It was prompted by noticing the many “hairs” on specimens that I had previously just ignored.
---------------------------------------------------------------------------------------------------------
US AND THEM: Like many parts of our anatomy, we mentally dump those small diameter strands of protein that occur all over our body into a basket called “hair”. We know that some keep on growing longer, some stop at a certain length, patches may fall out and cease to exist entirely, or we remove them by shaving or chemical means. Our pets, farm animals, and wilder neighbors possess these valuable small protein rods or tubes and use them for coats in winter, shedding them in summer; have several different types and colors in their seasonal clothes; create manes and tails which often continue to grow; form whiskers that are used to detect objects; and employ the ensemble for protection against abrasive skin damage and insect attacks. But insects! They are different, they are not like us! Well, maybe. Let us inquire. Arthropods have hair-like structures call setae. Photos 1-21 illustrate the points made in the following paragraphs. (In what immediately follows an older, simpler entomology anatomy is used. Where possible, terms that are not used subsequently appear as [italics] items.)
Cuticula and Chitin: One can detect, more or less, three distinct layers in the insect body wall. There is an outer protecting layer, the cuticula; an intermediate cellular layer, the hypodermis; and an inner delicate membranous layer called the basement membrane. The hypodermis is a living part of the body-wall. Certain of these cells become specialized and produce hollow hair-like organs which remain connected through pores in the cuticula. These specialized cells are called trichogen.1 Outside of the hypodermis is a firm layer that serves as a support for the internal organs, and protects the body. (Fig. A) The larger portion of this cuticula is formed from chitin, a horn like substance. Chitin is a polymer of 2-acetylaminoglucose; a glucose molecule where the hydroxyl (HO-) group on carbon #2 is replaced by an acetylamino [CH3CONH-] group. (Fig. B) Chitin was used by trilobites as a refracting interface to correct for the spherical aberration of the calcite-like dome lenses in its compound eyes. Chitin is often cited as the lenses of the compound eyes of Dipteran insects like the house-fly. And aminoglycan polymers occur in mammalian eyes, and are used for ophthalmologic purposes. Chitin forms the protective sheath in beetles, and the cuticula of other arthropods. Chitin even is used in the development of powerful teeth in some arthropods. The mandibles of a few beetles have a Mohs scale hardness of ~3, like calcite crystals, and they can chew on soft metals like lead, tin or copper. Chitin occurs in both flexible transparent or translucent forms, and a hard, dark rigid form. Protein components are often linked together by quininone copolymer linkages, resulting in a “tanned” brown material. Chitin is oriented in such a way that insect exoskeleton fragments show little birefringence. The chitin is laid down in parallel microfibers forming a sheet, and each successive sheet has its fibers on a bias with the previous layer. The muscles in your abdomen are similarly biased, since it gives added strength, just like biased layering in rubber tires. When flexibility is required, as in wing hinges or leg joints, an elastic rubber-like material involving coiled protein chains is present. The epidermis is the location of cuticular pigments. (Photo 1)

Figure
A Body wall, diagrammatic cross sectional structures;
Figures
adapted from Introduction to Entomology, J. Comstock, 9th
Ed., 1940
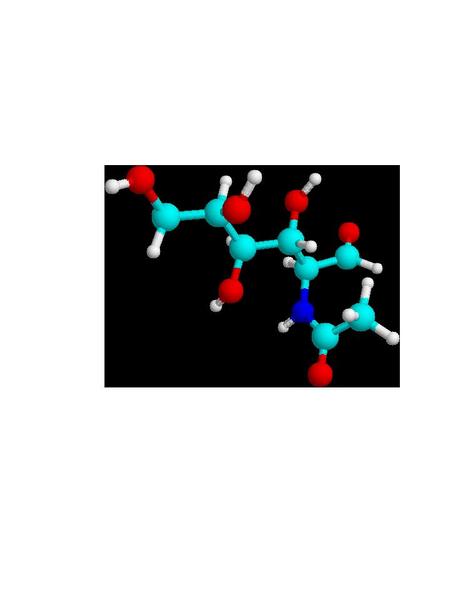
Figure
B 2-acetylaminoglucose, building block of chitin
light
blue=carbon, dark blue=nitrogen, red=oxygen, white=hydrogen

Photo
#1 beetle- chitin tanned sheath
PROTUBERANCES: The outer surface of the cuticle presents a magnificent variety of projections. Some are connected to the cuticula by a joint. Others form an integral part of the cuticula. Large projections are termed spines. These are of multicellular origin. Appendages on the legs of insects, but joint connected, are called spurs. The wings of some insects, like the Lepidoptra, also present large numbers of structures, in addition to normal setae, which are spatulate, or scale-like in shape, and are often highly colored.
Setae might be referred to as insect “hair”. Some deer’s hairs are hollow for insulation. Setae are also hollow, and associated with one single cell- the trichogen. Setae serve many purposes. There may be gland cells opening into the setae, or a nerve may extend into the hollow shaft, forming a sensory device—a sensillum (pl. sensilla). (Fig. C) In such cases the trichogen cell grows the conical hair, another cell [tormogen] grows the socket. A sensory neuron cell grows a dendrite into the hair and an axon extends inwardly to form a nerve connected to the central nervous system. Imagine a root canal on many of these on your next trip to the endodontist. In tactile structures the setae are relatively long. For taste, smell, temperature or other sensing they may appear as pegs, pits, buttons, or cones; and often several neurons and their dendrites/axons are present. The setae can also serve as chemical weapons, and let insects walk on water. Some of the setae serve merely as “clothing hairs”. The setae may be birefringent. The birefringence of insect setae is never as high as in cellulose plants, but it is higher than in the exoskeleton fragments.”2 (Photos 2-5)
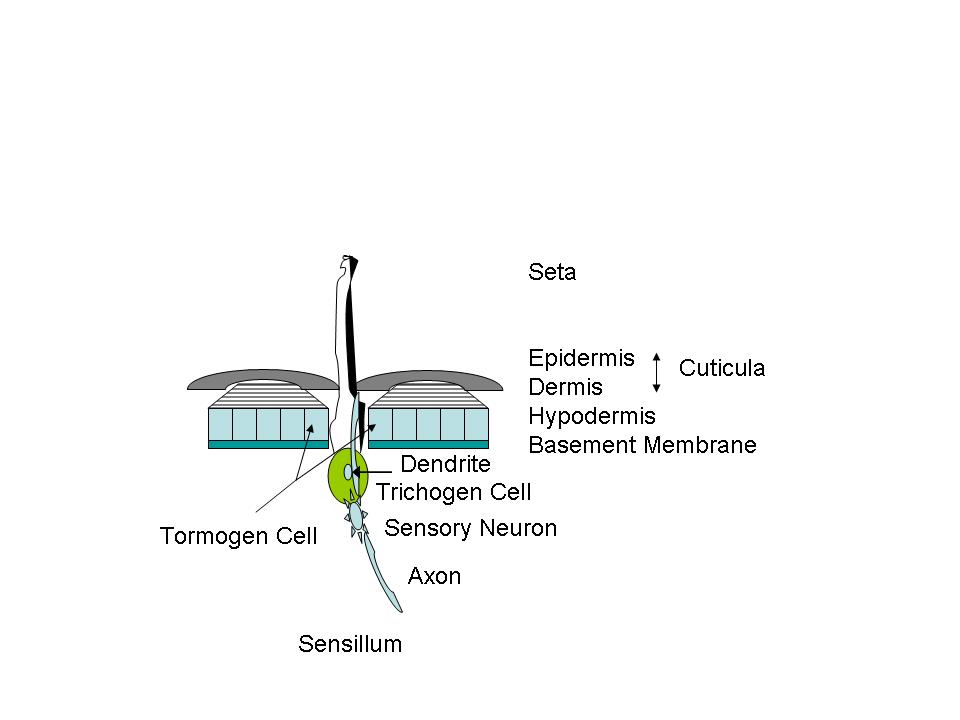
Figure
C Basic diagrammatic sensing structure

Photo
#2 setae

Photo
#3 setae foreleg
Photo
#4 setae tibia, tarsus

Photo
#5 tibia spines, foot-tarsomeres, setae, claw
Setae nerve polarization and depolarization, works like ours— that is, action potentials are generated in the dendrite (input side of a neuron), depolarization travels along the length of the nerve, and output sent along an axon (output side of a neuron). Depolarization causes neurotransmitters to be released into the connection [synapse] between the axon and dendrite. Common insect neurotransmitters include molecules [acetylcholine and catecholamines, like dopamine] that are used in human neurotransmision. Many pesticides act by interfering with the functioning of the nervous system neurotransmitters. The fundamental difference between invertebrate and vertebrate nervous systems is the number of cells: insects may have half a million neurons, while vertebrates may have 10 billion or more.
Grasshoppers, LOCUSTS AND CICADAS: The hind femur of the grasshopper is the enlarged jumping spring of the hindlegs. The hind tibia has two rows of spines and as many as six enlarged movable spurs at its apex. Note that a spur is inserted into a socket and is movable while a spine lacks a socket and is fixed. Stimulation of a single prominent sensillum (sensing appendage) on the hind foot [tarsus] of different species of locusts can activate the fast extensor tibiae muscle. The grooming reflex of the locust's front leg is mediated by hair sensilla of the sternum region. These hairs are ~ 50-200 um in length. Studies of electrical nerve pulse propagation after manual stimulation of an insect setae by touch of just a single human hair have been made.3 (Photos 6,7)

Photo
#6 grasshopper- spines

Photo
#7 cicada- transparent-wing, spines, spurs
Butterflies AND MOTHS: In moths the adult uses its thoracic legs for several types of behavior not displayed by the larva. These include walking with an alternating gait, grooming the antennae and mouthparts, landing on a substrate after flight, perching on a leaf during oviposition, and more subtle behaviors such as "tasting" the leaves of a host-plant. The adult leg has new structures such as tibial spurs on the thoracic legs. The tibial spurs on these legs contain scalelike tactile hairs, and chemosensory sensilla.4
In butterfly larvae, tactile setae are scattered fairly evenly over the whole body. You can see these setae on Monarch larvae with a simple magnifying lens. Larvae have a variety of responses to touch, like coiling into a tight spiral. Adults have tactile setae on almost all of their body parts. The setae play an important role in helping the butterfly sense the relative position of its many body parts (e.g., where is the second segment of the thorax in relation to the third segment). This is especially important for flight, and there are several collections of specialized setae and nerves that help the adult sense wind, temperature, and the position of head, body, wings, legs, antennae, and other body parts.5 The fringes or “feathering” on insect wings also help aid flight. (Photos 8-15)

Photo
#8 butterflies- wings, veins, scales

Photo
#9 moth- wing, scales
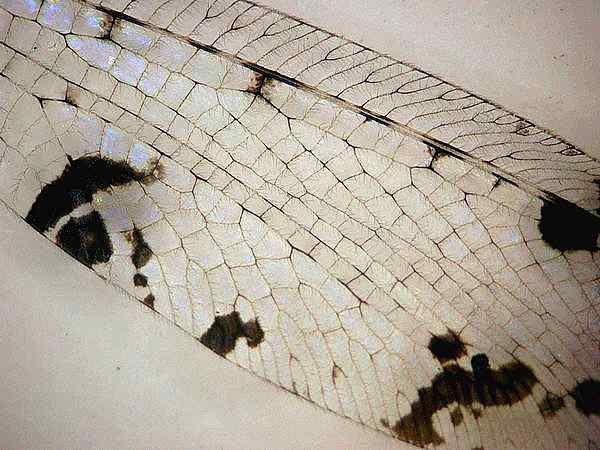
Photo
#10 transparent-wing, setae, veins
Photo
#11 moth- wing, veins, scales

Photo
#12 butterfly- wing, scales, color

Photo
#13 butterfly- wing spatulate scales

Photo
#14 moth-antennae

Photo
#15 moth- wing “feathered” edge, antennae
In some cases, like butterflies, setae and vein structures strengthen the wings. Butterfly and moth scales themselves are modified setae, overlapping pieces of chitin. Butterfly wings are made of two chitonous layers (membranes) that are nourished and supported by tubular veins. The veins also function in oxygen exchange ("breathing"). Covering the wings are thousands of the colorful scales, together with many normal hairs (setae). The name Lepidoptera (which includes butterflies and moths) means "scale wing" in Greek. The colour of the Eurema lisa butterfly originates from light diffraction. The bright colour of the Morpho butterfly originates from light interference and diffraction, and the blue colour of the Polyommatus daphnis is caused by photonic crystal effects. A beautiful TEM and SEM study of some butterflies has recently appeared.6 The scales are usually arranged regularly from the front to the end of the wing just like the tiles on the roof. The scales are all tilted and have the same angle to the wing plane. Each single scale is likes a tiny shield with 50 to 80 um width and 150 to 200 um length. Our optical microscopes can hint at their beauty.
Multidendritic sensilla occur in antennae. A male silkworm moth has ~17,000 sensilla, each containing up to 3000 pores, 10-15 nanometers in diameter. Less than a hundred sex pheromone molecules could initiate an upwind behavioral response. Human fashion perfumes and noses just cannot compete.
Caterpillars: Caterpillars sense touch through tiny hairs (setae) that are all over the caterpillar's body. These tactile hairs often grow through holes in the dark, flattened plates on a caterpillar's body. These hairs are attached to nerve cells, and relay information about touch to the insect's brain. (Photos 16-18)

Photo
#16 larva- setae

Photo
#17 caterpillar- setae

Photo
#18 caterpillar- setae
But caterpillar setae are also the source of defensive mechanisms. Some involve chemical toxicity, while others are “cloaking” in nature, making it difficult for parasites or predators to deposit, inject or attack. Recently a new effect has been seen in Mare Reproductive Loss Syndrome (MRLS). Having an interest in breeding horses, it is noted that our neighboring state of Kentucky has reported 60% or higher early loss of foals after birth. Losses in the vicinity of $400,000 occurred. Studies showed that the losses resulted from ingestion by the pregnant mares of Eastern Tent Caterpillar bodies. Autoclaved caterpillars did not result in MRLS, but frozen specimens did. The white, dense “tent” material had no effect. The foals died of bacterial infection associated with co-habiting [commensal] organisms that occur in the mares mouth and GI tract.
One current explanation is that the hollow setae serve as well-protected hypodermic syringes that penetrate the gums and intestinal wall of the mare, and migrate to receptive sites like the uterus, with the bacteria lodged in the lumen of the setae shielded from normal antibody reactions. Although the “Septic Penetrating Setae” hypothesis has yet to be experimentally confirmed, many people are also affected by irritation and allergic responses due to handling caterpillars indiscriminately.7 Handling hay on hot summer days is prickly too.
Spiders, Setae, and Prey: Setae in spiders certainly are used to provide a sense of touch, and they are involved in proprioception. The latter allows an animal to determine where its various body parts are deployed during movement. Is this important? Years ago, before a visiting lecture, a colleague asked me to taste a leaf—deliberately neglecting to tell me that it anesthetized the proprioceptors of the tongue. Try talking when you don’t know the shape and position of your tongue. Beautiful arachnid setae SEM photos are available8, but optical microscopy can reveal some of their secrets. (Photos 19, 20)

Photo
#19 orb-spider- limb setae

Photo
#20 spider- body, leg setae
A fascinating WMD (weapons of mass destruction) story surrounds the size, shape and positioning of insect setae, compared to the nature of the web of preying spiders. Some of the latter are “primitive” and consist of fibril populated threads with thousands of fibrils that are ~20 nm in diameter. They can entangle setae temporarily, allowing the quick spider to gain its prey. Other webs have viscous sticky droplets periodically arrayed on the thread, with different species having droplet volumes that range from 0.1-10 um3, and with 25 to 5 drops/mm. “The volume of viscous material per mm of thread length differed by as much as 22-fold among the four spider species’ threads studied. The length of setae on the four insect surfaces studied differed by as much as 230-fold and their densities by as much as 7170-fold. …
The study showed that the surface features of an insect body determine how much of a capture thread's potential adhesion contributes to insect retention. This operational thread adhesion combines with features of web architecture, such as capture-spiral spacing, and with a spider’s running speed and mode of prey immobilization, to determine how securely insects are held by a web and which are most likely to be captured by a spider.”9 The positioning angle and shape of its setae can determine the destruction or survival of the insect. Many publications focus on the combination of van der Waals’ forces and capillary attraction forces in any adhesion process. The van der Waals’ forces arise when molecular entities come into close proximity, and the charges of one molecule are attracted by charges in the other. These are weak forces, but if there are large numbers of liaisons and enforced intimacy the result can be significant. Capillary attraction is associated with the surface tension of water and the wet-ability of the surfaces. Cohesion can be formidable. If you have a hot/cold faucet with just one control handle it may involve two finely polished ceramic disks mounted concentrically on a rod with each disk having holes along a radius. A very thin water layer between the disks can create sufficient capillary adhesion to withstand the pressure of the water mains and keep the faucet from dripping. Pulling the disks apart manually requires strong muscles. Viscous threads seem to need a relative humidity of ~ 45% for this effect to be active. The nature of the glycoproteins in the droplet is also important.
Walking on the Ceiling, Walking on Water: The feet of some insects, and hunting spiders, are often covered with setae that branch out into smaller fibers [setules]. With miniscule van der Waals’ forces acting on large numbers of these small fibers on each furry seta, an arthropod can hang and walk upside down on a leaf or a wall with ease. Capillary adhesion is also usually invoked. The gecko is often mentioned in association with these phenomena. Every square millimeter of a gecko's footpad contains about 14,000 hair-like setae. Each seta has a diameter of 5 microns. Each seta is in turn tipped with between 100 and 1,000 spatular fibers. Each spatula is 0.2 microns long, or near the wavelength of visible light. Recent publications suggest that van der Waals’ forces and capillary adhesion are involved.10 You decide between the rivalry! Someday you might buy rolls of “Gecko Tape” since engineers would like robots that can climb walls.
Setae also aid some aquatic insects in locomotion and enable them to “walk on water”. Many “brushlegged” caenid larvae have extremely long and profuse hairlike setae present on the legs11. Yet the adults swarm in hordes and lie, dead, floating flat in our horse trough. But butterflies somehow avoid sticking of their wings; and water striders float and move on a water surface. “The natural super-hydrophobic surfaces generally have three common features: (a) they are coated by wax or a hydrophobic film; (b) they are decorated by textures such as bumps, pillars, or grooves at a scale of typically a few micrometers; and (c) they have a secondary texture superposed on the first one at the nanometer scale. Besides surface chemistry, the surface roughness and geometry have a crucial role in affecting the super-hydrophobicity. Recent studies suggest that the super-hydrophobic property of the water strider’s legs is due to the long inclining spindly cone-shaped setae at the surface.”12 First, the air trapped in the cavities and texture features of the super-hydrophobic surfaces can provide extra buoyancy forces, which can reduce the apparent density of a water strider to 0.71 g/cm3 in water.13 Second, the water strider leg owes its hydrophobicity to its complex surface cover of hairs coated with water-repellant cuticle wax and contoured with fine fluted nanogrooves.14 Water-walking insects [e.g., Microvelia and Mesovelia] generally keep three legs on the water surface at all times in order to maintain static stability. The exception is the water strider, which generally keeps its pairs of front and hind leg tips on the water surface while its middle legs row. (Photo 21) In this photograph the circular dark shadows on the stream bottom are consistent with the divergent lensing effect of the dimples created in the water surface by the insect’s super-hydrophobic feet.15

Photo
#21 water-strider- “feet-shadows” on stream bed
(Consistent
with divergent lens images from water-surface dimpling)
And some insects use chemical propulsion. This means of propulsion [Marangoni Propulsion] is used as an escape mechanism by a number of water-walking insects, as well as beetles and terrestrial insects that accidentally fall onto the water surface. They release a lipid that reduces the surface tension behind them, propelling themselves forward at peak speeds of the order of 20 cm/sec..16 When a pine needle falls into a lake or pond, it is similarly propelled across the surface since the resin at its base decreases the local surface tension. Imagine fabrics that are super-hydrophobic, or something that you could paint on your feet to make swimming easier. Unfortunately, humans are too heavy, and the Olympics’ Committee would forbid it.
Insects created a nano-world first, and imagined a “better living through chemistry” long before we began to imagine our superiority.
Photographs: The macro-photographs were largely taken with live subjects, either in the field or captured and temporarily cooled to 50°F to reduce motion. The camera was a Nikon 5700 with extended zoom [250 mm (35 mm eq.)] and a 4 diopter or 10 diopter close-up lens. Maximum magnification was 1 to 2X. Natural lighting or clip-on reading-flex-lights with high intensity Nichia LEDS were used for front illumination. Backlighting employed a fluorescent “trouble-light” housed in a flat translucent plastic container. Zoom-stereo photos employed the Nikon 5700 with a Martin Microscope relay lens attachment inserted into one of the binocular ports of an American Optical 1-4X objective stereo microscope. Magnification was 12-50X. Lighting was by a 48 LED ring-light with pulse modulated intensity control obtained from Martin Microscope. Microscope photos used a modified BH Olympus Scope with incandescent normal and epi-illumination capability, and the Nikon 5700, with relay lens attachment, inserted in the third camera port. Full extension zoom, infinity focusing lock, and manual infinity adjustment was used. Preset white balance was employed. Magnifications of 60-120X were employed. Focusing was aided by an LCD video display attached to the camera output. All photos are uncropped, and adjusted only for normal LCD monitor gamma requirements.
Comments to the author Ray Dessy are welcomed.
Acknowledgements: Thanks to Eric Day, Entomology Dept., Virginia Tech, for his help in focusing on the subject. Compression, editing, and organizing of the photographs was done with the free downloads of PhotoFiltre, FastStone Image Viewer, and Picasa (Google).
References (Click each ref. number to return to respective article text.)
1 P. Gullan, The Insects; An Outline of Entomology, 2005; C. Gillot, Entomology, 2005
The simple cross sections used are from an older text; J. Comstock. An Introduction to Entomology.
2 MicrlabNW, Web Site collection of insect photographs Insect%20Hair_MicroLab_files/InsectHair7A58807.jpg
3 H. Pfluger, J. Exp. Biol (1980), 87, 163-175
4 K. Kent, Cell Tissue Res (1990) 259:209-223
5 www.MonarchWatch.org monarch@ k u . e d u
6 L. Wu , Journal of Bionic Engineering 4 (2007) 47-52
7 Immunoblogging%20Mare%20reproductive%20loss%20syndrome%20and%20caterpillars.htm
8 I. Agnarsson, 2007 (2008) The Journal of Arachnology 35:411–426
9 B. Opell, The Journal of Experimental Biology 210, 2352-2360 2007
10 K. Autumn, Proc. Nat. Acad. Science, 99,12252, 2002
G. Huber, Proc. Nat. Acad. Science, 102, 16293 2005
11 A.V. Provonsha, Journal of Insect Science: Vol. 2006 | Article 10
12 Y. Ding, Nanotechnology 19 (2008) 35 5708
13 X. Ziang, J. Mater. Chem., 2008, 18, 621–633
14 J. Bush, Annu. Rev. Fluid Mech. 2006. 38:339–69
15 See in vitro photos by L. Jiang, Nature 432 36
16 J. Bush, Advances in Insect Physiology, Vol. 34, 2008
NB: Many of the above journals and books may be a challenge to access. Although most of the references are in traditional bibliographic form, since URLs often change or disappear, much of the bio- and entomology sources are available on-line. The workers feel information should be open-access, and many government agencies insist on free Web access 6 to 12 months after print publication if federal support is involved. Searching for author, journal and a few key words may obtain access to the full reference. Please e-mail if help is needed in your exploration.
Published in the January 2009 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
with full
mirror at
www.microscopy-uk.net
.