
|
The Nightmare of Classifying Protozoa (and Other Protists) by Richard L. Howey, Wyoming, USA |
Take a drop of water from a nutrient rich pond or “wild” culture, place it on a slide, and you are ready to enter an alien micro-zoo or botanical garden if you happen to have found an algal forest. If you are showing such a sample to a beginner, one of the very first questions you will hear is: “What’s that?” So, you take a quick peek through the microscope and see 30 different kinds of organisms and you ask: “Which one?” realizing that, of course, the critter in question may have moved out of the field of view by this time. This now becomes a test of the powers of observation and description of the beginner–an experience which, in one form or another, we have all gone through and which, in a very significant sense, is a never-ending process. After over 65 years of being a protistic voyeur, I still regularly encounter organisms which are unfamiliar to me.
If your interest persists and you begin to get serious about studying protists and classifying them you will encounter a bewildering series of complex, challenging, and fascinating problems. At the time, they will seem absolutely overwhelming, but when you resolve one difficulty after another, there is a sense of genuine satisfaction accompanied by a recognition that even the most eminent researchers are still wrestling with many of these problems. (The only real difference between us and them is they have grants, all sorts of high-tech toys, and laboratory assistants.)
Working with taxonomic keys can be extraordinarily frustrating, since not uncommonly the criteria given at a particular step can be ambiguous and a decision may depend upon highly technical terminology. Almost inevitably when trying to use a taxonomic key, I drag out my dictionary of biology to consult as I struggle through the key.
What I am going to attempt to do here is to raise some very basic questions that arise in classifying protists and, at the same time, show how and why certain stubborn ambiguities present themselves. In the early days of microscopy, in the late 18th and 19th centuries, there was much greater freedom with regard to naming micro-organisms. Sometimes, the names assigned were descriptive which can be quite helpful. For example, Paramecium comes from the Greek paramekes which has the roots “oval” and “long”. Then someone noticed quite a large species with a number of micronuclei and we get Paramecium multimicronucleatum. So now, if you have the right stains, a good microscope, and some Paramecia, you can find out whether it’s that species or not. The other general tendency during this period regarding the naming of organisms had to do with an element of the human psyche–egotism. In the early 19th Century, I could very likely have gone out to the ponds, brought back a sample and found a new species of Paramecium and called it Paramecium howeyii, thus achieving immortality, even if it be a rather feeble sort, perhaps even-oxymoronically temporary immortality--since, there was always the possibility that some other investigator might come along and show that what I thought was a new species was just a variant of Paramecium caudatum. Goodbye immortality!
Everything was further complicated by the fact that there was no internet and so, the same beastie might tend to show up with 6,7,10, or 12 different names, because publication was a slow and laborious process and the latest copies of your microscopy and natural history journals were delivered by horse and carriage. This problem about a lot of different names has long been recognized; it is the problem of synonymy.
On top of this, there was, and still is, the problem of language. If my German, French, Italian, Polish, Russian, Danish, and Swedish were a bit rusty, I might miss out on some important papers on the organism I was researching and only a year later would I find out that some Russian had renamed Paramecium howeyii and called it Paramecium smerdyakovii. All of this became such a nuisance that gradually a set of international rules developed for naming organisms. The Internet represents a giant step toward beginning to resolve some of these difficulties, but it will take a lot of time and money, because there are many articles in obscure journals in languages which relatively few people, on a global scale, know. Translations by humans are very expensive and computer translation is still in its infancy.
A further complication is the fact that professional biological scientists, more and more, face the demand that their work have practical applications in medicine, bio-technology, biological warfare, or industry; otherwise they find it very difficult to get funding. This means that “pure” research, the non-practical, exciting stuff is left more and more to the amateurs. I knew a cell biologist who for 30 years never went out to the field to collect any of the organisms he was studying; he relied on someone to supply him with the appropriate species and it was important for him to have certain definite species, so that other researchers might verify his work.
So, how does an amateur, who is seriously interested in protists, begin to cope with the labyrinthine complexities of taxonomy? My recommendation is that one should begin by asking oneself a series of basic (often, not so simple) questions.
Let’s imagine that we have an organism in the cross-hairs of our microscope.
1) Is it moving?
a) Yes. (This may simplify things; then again...) Go. To #2
b) No.
“No” presents a series of possibilities.
b1) Is it really alive or just a bit of detritus?
b2) Is it a non-motile algal form? (And remember that not
all algae are green.)
b3) Is it a dormant stage of some protist that is sometimes active?
b4) Is it a cyst?
Also, remember that even if it is moving, you can’t assume that it is a protozoan: it could be an algal form or even a cyanobacterium such as Oscillatoria.
2) If it is moving, can you detect its means of locomotion?
a) if the answer is “Yes”, we again confront a series of possibilities.
a1) Does it move by means of flagella (undulipodia)? If so, it is probably a flagellate. I can hear you saying: “Well, DUH! That’s pretty obvious!” Well–not necessarily. There are at least two intriguing groups of flagellated amoebae in addition to certain flagellated stages in the complicate life cycles of foraminifera.
a2) Does it move in an amoeboid fashion which can be surprisingly quick in some species, as slow as molasses in others, and in jerky bursts in yet others? The probability is very high that it is an amoeba, but it could be Trichoplax or some other highly bizarre beastie.
a3) Does it have cilia? Well, if we assume that it’s not a larval form of a higher organism from another kingdom, then it’s probably a ciliate, but for an amateur trying to learn classification criteria, that’s not a very helpful assumption. So, at this point we’re in the position of saying that we have a ciliated “something” which might be a protozoan.
2b) No, if the answer is “No” that we can detect no means of locomotion, then again we have several possibilities.
b1) It was motile, but now it’s dead.
b2) It was motile, but now it’s resting or dormant or encysted.
b3) It lives in a “house” or shell or test or lorica and is temporarily inactive. Interestingly, amoebae, flagellates, and ciliates all utilize this strategy in different forms.
Amoebae tend to be the real builders. Forams build elaborate, chambered, calcareous tubes or lovely spirals and some even look like Belleek vases. Radiolaria are the aristocratic architects of the micro-world. They extract silica (essentially, glass) from seawater which is a rather amazing feat to begin with and then they create phantasmagoric “mansions” in the form of spheres, “space capsules”, pyramids, and wheels, all of which are punctuated with hundreds of pores, allowing for the projection of protoplasmic pseudopodia to create a series of organic nets which are very effective at capturing prey. However, most of us are generally able to view only the tests or “shells” which are in themselves minor miracles of nature.

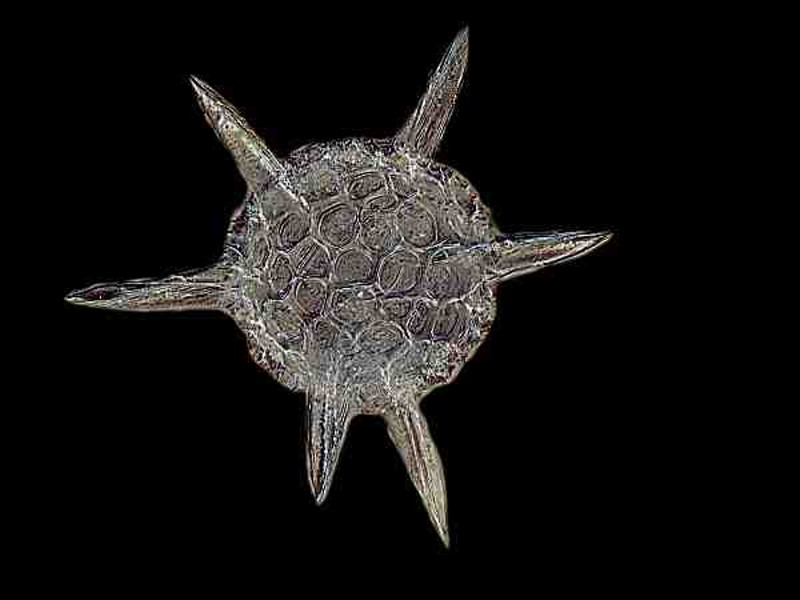

The shells themselves are often decorated with spines and knobs and look like hi-tech communication satellites. In the living state, some create a cushion of bubble-like protoplasmic projections (alveoli) around themselves. There are also the less imaginative sorts of amoebae which tend to use items conveniently at hand. Various species of Difflugia utilize sand grains and some species may also decorate a bit by using some diatoms or desmids. Other species are quite fastidious and pick sand grains that are all about the same size and neatly polished. Yet others build in the way I would if faced with such a task–a style which I call neo-sloppy–by taking any sand grain not too wieldy and sticking it on. Yet another group of amoebae, Euglypha being an example, secrete elegant overlapping plates. And the there are the Arcellids which are rather cute with their flattened, hemispherical, chitinous shell with its pseudopodia peeking out from below when it’s moving. When Arcella divides, the daughter cell has to secrete a new shell which initially is so thin as to be virtually transparent. As the organism ages, the shell becomes tan and translucent, and in its “old age” takes on a deep rich brown color and is opaque.
So, amoebae have a lot of protective strategies, some of which make classification easier, at least at the general level, but sometimes more complicated at the species level, since some species can show considerable variation.
Flagellates are also tricky. Sometimes you will come across a tiny vase-like structure, frequently attached to a plant stem and it may or may not have a stalk. You may not observe any activity at first but if you are patient, you may begin to notice movement within the tube and then suddenly a couple of flagella may appear extending beyond the collar or vase, whipping the water and creating micro-currents to bring in even smaller organisms to feed on. Sometimes these “collar” or choanoflagellates occur in clusters. Interestingly choanoflagellates are a basic sort of organism comprising sponges. This raises wonderful sorts of problems about the colonial organisms and the current, nasty, politically incorrect “E” word–“Evolu...” Or dare I spell it out–yes, “Evolution”–there!
Flagellates quite often occur as “colonies” ranging from 4 “cells” to thousands. The reason for the quotation marks are: 1) the concept of colony is notoriously ambiguous and vague and I hope soon to write an essay on this subject and 2) the designation of cell is generally inappropriate when applied to protists since these are complete self-contained organisms.
Gonium sociale is an example of a colony of four and there are other protists with 3, 8, 12, 24, 48, and on up to thousands of “cells” in Volvox globator. The larger “wheel” or sphere colonies of flagellates are extraordinarily striking as they gyrate through the water. Sometime you see a brownish spherical colony rotating through the water and as you track it, you may very well come across a brown, branched ropy-looking “tree” with the very same sort of colonies attached to the outermost tips of branches. This is Anthophysis vegetans and though I have encountered it numerous times, I am still completely puzzled regarding how these organisms secrete, produce, and construct their “tree”. Here you can find some images of this bizarre organism.
Ordinarily, I don’t mention parasitic organisms because I rarely work with them and then only when preserved or on prepared slides. However, there is a group of flagellates that have produced a remarkable structure that is worth, at the very least, a passing mention–the trypanosomes. These are blood parasites and have a significant impact on humans both directly and indirectly. Perhaps the most notorious ones are those that produce “sleeping sickness” in humans. However, there are others which take a significant toll on livestock and cause economic crises for tribes dependent on such animals. This problem can extend from the tropics all the way to the Arctic, since certain trypanosomes infect caribou. The reason that trypanosomes are of morphological interest here in this discussion is that a flagellum has been significantly modified to form a boundary along an undulating membrane. These organisms can occur in staggering numbers nearly equaling the number of blood cells. The membrane is visible in well-prepared, stained preparations.
Fewer types of ciliates build houses because generally they are a fairly active group of predators. However, there are some interesting exceptions both in fresh and marine waters, such as Vaginicola and the tintinnids.
Diatoms and desmids can be observed moving as well and biologists have wrestled with the means of their movement for over a century and a half. Fortunately, the slow gliding manner of the motion coupled with other distinctive features makes them quite easy to recognize as diatoms or desmids but, after that, getting to a species level or even a genus level may require special expertise and equipment.
As you can see, classification is enormously complex and modern biologists insist that in some instances only by using high-tech tools, such as scanning and transmission electron micrography and biochemical analysis can species be definitely determined. The amateur out of frustration may be inclined to ask: What difference does it make? If we’re dealing with deadly pathogens, particularly those that have a high capacity for rapid mutation, it can make a difference between life and death. However, for us as amateur investigators, those are not our issues, but it does help us understand why contemporary taxonomy has become so complicated.
Let’s go back to the issues of movement for a bit. There are protists with forms of motion that are so distinctive that it makes them rather easy to recognize, at least at the genus level. Let me give you a few examples. When you see colonial flagellates rolling through your field of view, that helps narrow things considerably. Sometimes, “mere trifles” can be of help in identifying an organism. There is a colonial flagellate called Synura uvella which in spring may occur in enormous numbers giving the water a distinct cucumber-like smell.
Amoebae exhibit a few basic types of locomotion. Just as an aside, let me remind you of something you already know, but that we are all prone to forget at times–amoebae are 3-dimensional creatures. If you find some large ones, put them under your stereo-dissecting microscope and let them adjust for a few minutes. Then, if you’re lucky, you’ll find a few exhibitionists who will extend their pseudopodia straight up at you as you observe them.
Some of the large amoebae can move at surprisingly rapid rates, enough so as to sometimes make it difficult to get a good image of them. Others, particularly certain monopodial forms, will extend their “foot” and then there is a pause, followed by a sudden surge like a miniature dam bursting, followed by another pause. This typical form of amoeboid locomotion can be seen in the Thecamoebae and it is almost like a combined stretching and crawling. These images, however, show the organism in a resting state and it is quite clear that they have just had a large festive meal which they are in the process of digesting.
In the ciliates, we find the greatest variety of forms of movement, some of them associated with locomotion and some not. Because of the ubiquity of Paramecium its movement has, I suppose, come to be taken as a kind of model for ciliates. Paramecia, when feeding around a clump of bacterial detritus, tend to dart in and out of the bacteria-rich areas, often in a rather jerky fashion. When swimming in open water, they move sleekly, often rotating as they propel themselves. Their behavior demonstrates the efficiency of their hydrodynamic design.
However,, ciliates have a number of other clever tricks for getting around. Halteria grandinella has, around its midsection, a number of long, spine-like structures which appear to act like springs and often just as you are focusing in on a specimen, it will go bouncing our of your field of view. Then there are the giants like Spirostomum ambiguum which slowly glide through the water like micro-whales, but which when disturbed, contract to about 1/3 of their full length in an astonishing 10/1000ths of a second and, at one time, this was claimed to be a record speed for contraction in any organism or cell. Stentor, especially the lovely blue-green Stentor coeruleus, is also highly contractive, but it has another trick as well. It has a hold fast and mucocysts from which it secretes an adhesive allowing it to attach itself to a substrate or other suitable surface. It is only then that it achieves its full extension, sometimes over 1500 microns and then actively feeds. If conditions become undesirable, it can detach itself and swim off to find better circumstances.
There are other ciliates that employ the strategy of attachment, some on a more permanent basis than others; in particular, the peritrichs, such as, the Vorticellids, and the suctorians. Vorticella was described by Leeuwenhoek who characterized it as a “horned animalcule”. You can understand how this came about by watching a Vorticella when its is feeding. The oral cilia move so rapidly that you can only see them at the opposite ends of the “bowl” of the body, thus giving the impression of minute “horns”. In addition to the ciliary activity, there is another radically different form of movement which Vorticella is capable of. Remember it is shaped like an upside down “bell” or “bowl” at the end of a stalk.
When disturbed, this stalk contrasts quickly by coiling. If you adjust contrast carefully and look closely at the stalk, you can see a fibril running through it. This is a spasmoneme (myoneme) which is akin in function to a muscle fiber in larger animals.
As if this isn’t remarkable enough, there are “colonial” vorticellids which have stalks branching off of the central stalk and in Carchesium each individual has it own independent spasmoneme so that each one can contract separately. However, in Zoothamnium the stalks are interconnected so that if one contracts, the entire colony contracts. Seemingly nature never tires of experimenting and, as a consequence, the attentive naturalist never runs out of wonderful surprises. Clearly vorticellids don’t move around much except incidentally when they attach to a copepod or a snail shell, but they do produce a “larval” ciliated state which swims about feeling until it finds a place to settle. It is called telotroch.
Even more charmingly bizarre are the suctorians. They too are attached, often on stalks, but in the adult state have no cilia–instead they have tentacles! At the end of each tentacle one typically finds a small disk which is quite sticky. I have seen ciliates with a body size equal to or larger than that of the central body of the suctorian, stuck firmly to the tentacle disk struggling to escape. The suctorian is able to penetrated the membrane wall of its prey and then, as though it were enjoying a soda through a straw, sucks the protoplasm out of its victim through the tentacle. Suctorians have cilia only in their larval stage.
Here a brief digression is in order. Generally we think of protozoa reproducing by division or conjugation followed by division or by the production of larvae, but the phrase “giving birth” is not something that we associate with protozoa. However, there is an order of suctorians, Evangogenida, that forms a small sac-like structure that inverts to push its larvae through a tiny “birth pore”.
Another ciliate with distinctive locomotive behavior is Urocentrum turbo. Its shape is basically an elongated oval with a slight indentation in the midsection with the top part being somewhat larger than the bottom. Both the anterior and posterior “segments” posses a band of cilia. However, even more interesting is a long bundle of cilia extending from the bottom of the posterior section rather like a tail. It secrets some kind of adhesive which allows it to “glue” itself to the substrate and then, the action begins. This remarkable creature begins to rotate like a miniature turbine and often, just as you are beginning to observe this amazing behavior up close, Urocentrum spins away out of your field of view like a tiny, toy top.
I have often thought that it would be nice if protozoans came color-coded for easy classification and, although that wish is fanciful, there are a few which do have distinctive pigments. I already mentioned the striking blue-green pigment of Stentor coeruleus (called “stentorin”), but this pigment has the additional feature of being dichroic; that is, depending upon the angle of illumination, this organism can show one of two colors, either the blue-green or a light rose color. There is another species of Stentor that is rose-colored, but it is smaller, more slender, and its pigment is not dichroic.
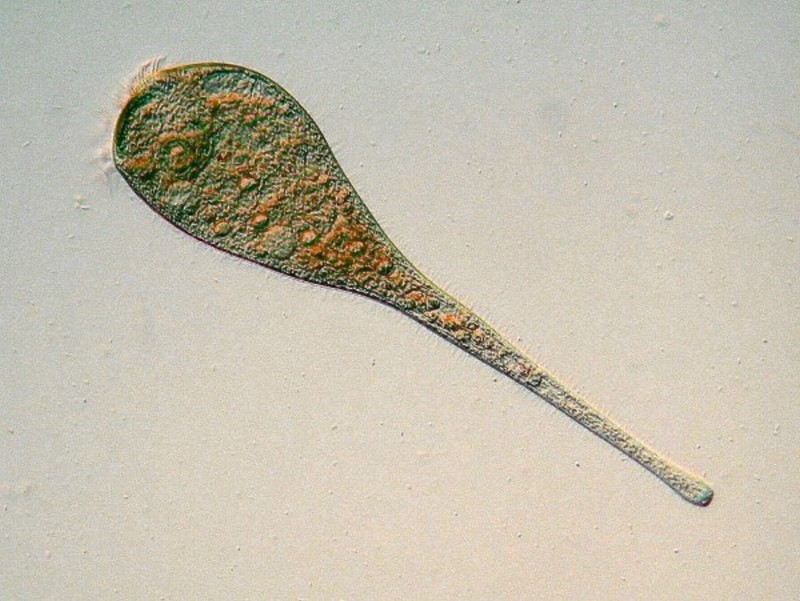
The image below is a closeup which shows the spiral arrangement of the fibers which allow for contraction and extension and also shows the chain or “beaded” macronucleus.
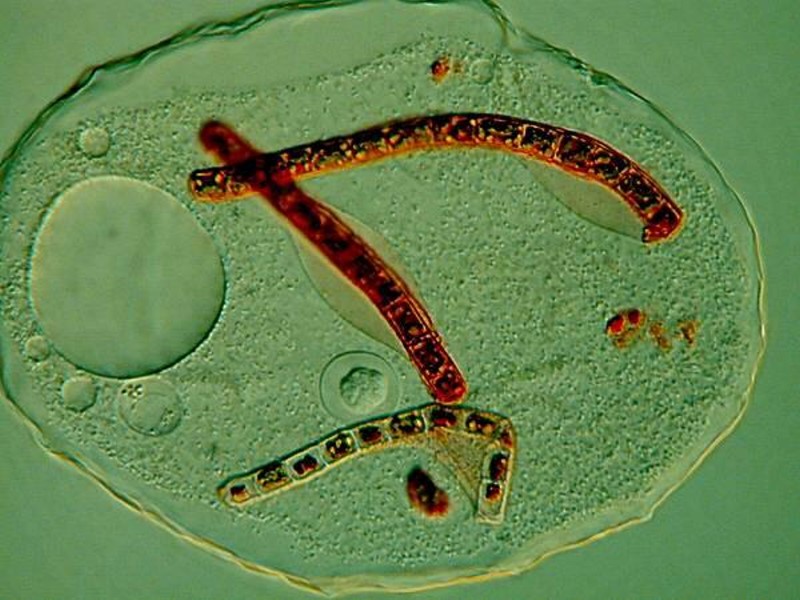
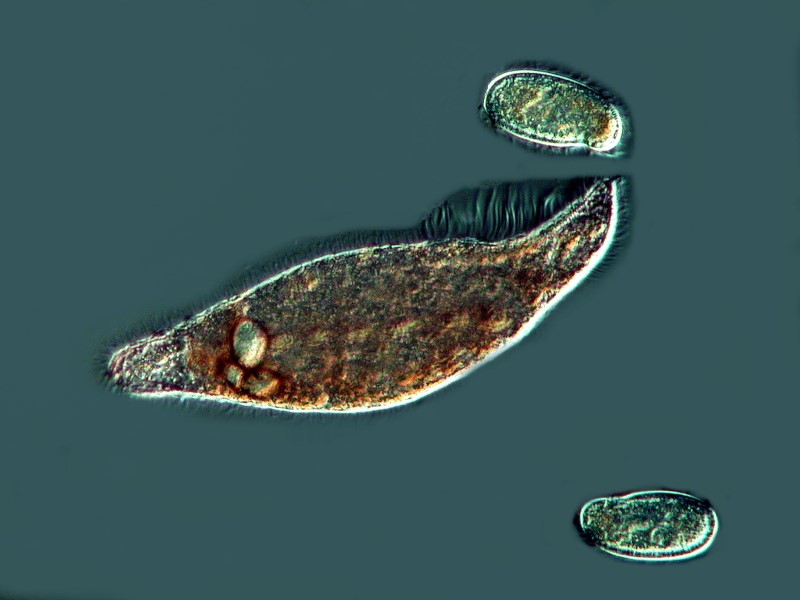
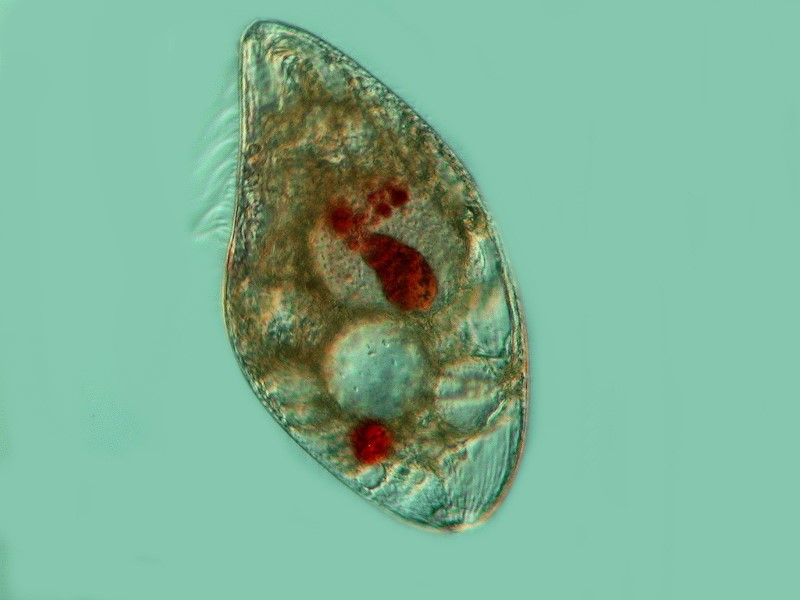
Another pigmented marvel is the sizeable ciliate Blepharisma which has a distinctive pink pigment which is unique and, as a consequence, has been given the name of blepharismin. Blepharismin is a photoactive pigment which becomes toxic to the organism in intense light. Presumably, one of the functions of this pigment is to help orient Blepharisma within the water column in areas that are well-lit, but not overly bright, in order to facilitate feeding, since many other micro-organisms are abundant at these light levels. This image shows the undulating membrane and 2 specimens of a smaller ciliate called Colpidium.
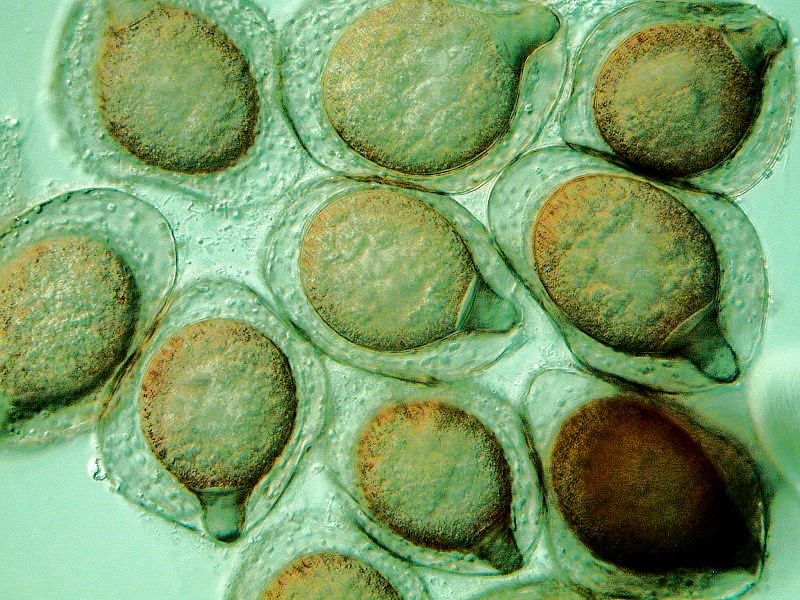
Under adverse conditions, Blepharisma forms distinctive cysts which you can see below.
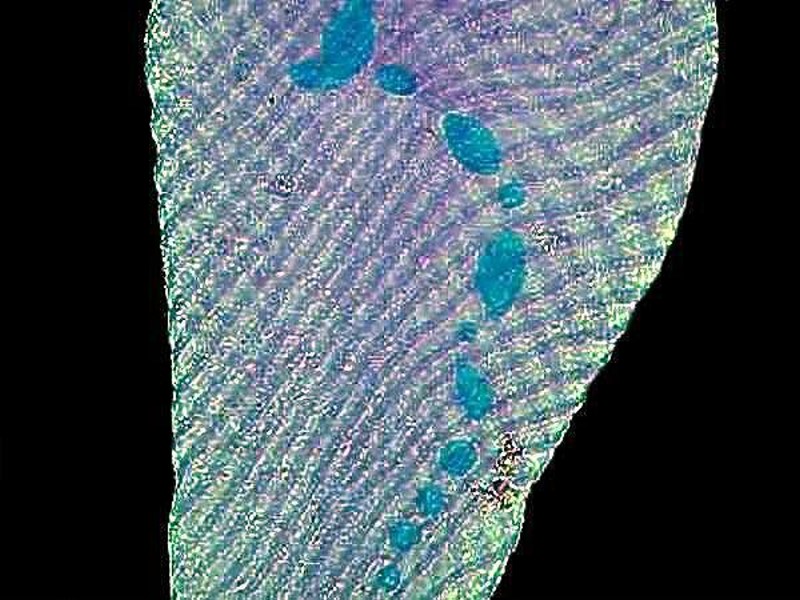
Blepharisma possesses what is called a “silverline system” which is revealed by using special techniques which involve the use of silver solutions as stains. This is a specimen which was stained using Protargol.

Blepharisma demonstrate a very unusual behavior, apparently when conditions become unfavorable for prey organisms, but this is not entirely certain. The unusual behavior is cannibalism and leads to the formation of “cannibal giants.” Below, you see a specimen which has ingested at least 3 of its fellows and the concentration of the pigment in vacuoles increases the intensity of the color.
There is a large number of protists which are green, containing their own chloroplasts or possessing symbiotic algae which have their own chloroplasts. Chlorophyll is a pigment which demonstrates primary fluorescence. This means one doesn’t need to use fluorescent stains to observe it. If you are fortunate enough to have a fluorescence microscope or know someone who does, you can observe, say in a desmid, the dramatic shift from the deep rich green color of the chlorophyl under ordinary illumination to a vibrant deep red under fluorescence. Pigments in protists are a marvelously complex business. The green are predominately chlorophylls, but as you might expect, there is more than one type of chlorophyll, the browns and yellow are usually xanthene pigments, and the reds and oranges are generally carotenes. To make it all the more fun, it is not uncommon for there to be a mixture of these pigments in a single organism or part thereof. Just think about the dazzling color shift we see in various kinds of autumn leaves. During the height of summer, when the leaves need to be food factories photosynthetically, they are rich in green chlorophyll. As autumn frosts arrive, the picture changes dramatically. As the chlorophyll cells die off, other pigments already within the leaves become visually dominant. The green cottonwood leaves become golden yellow and those of the quaking aspen can paint the mountainsides with great splashes and fingers of rich gold or brilliant orange.
Then, of course, there is the gorgeous array of reds, such as one finds in certain maples, and these are the consequence of carotene pigments. It is, of course, not the case that in autumn these pigments are new and magically appear in the leaves; they have been there all along, but masked by other pigments, predominately chlorophyll. But what does all of this have to do with protists? Interestingly, pigments of all three of these groups show up in a variety of protists and sometimes more than one pigment. One striking example takes me back to my youth, when one summer I was working on my great uncle’s farm during harvest. There had been some fairly heavy rains, so there was some urgency to get the harvesting done. Many small pools had formed around the farmyard and like most farmyards, it was rich in organic waste. One morning, as I walked out to the fields, I noticed a sizeable pond with a marvelous green scum on it–marvelous from a young, amateur microscopist’s point of view. Later, as we left the fields to return to the farmhouse for lunch–roast beef, ham, fried chicken, mashed potatoes with gravy of a sort that modern cooks can only dream of, freshly-baked bread, and cherry, apple, mince, and pumpkin pies–I paused by the same pond and was astonished to see not the rich green surface of the morning, but a dark blood-red scum covering the surface. My curiosity was excited but, for the moment, my great aunt’s cooking and baking won out. After lunch, I asked her if she had three small empty jars I could have and indeed she did. I was going back home the day after next, so that even after the sun had gone down, I took my first sample from the pool. The next day I took a sample when the surface was green and later another one when the surface scum was red. I couldn’t wait to get these home to my microscope.
With great anticipation, I put a drop from the night sample on a slide. I was rather disappointed to find the sample consisted almost exclusively of a medium-size, rather pudgy Euglena in enormous numbers. A drop of the red sample–same Euglena. Gradually it dawned on me that what I had seen in the barnyard must have had something to do with the light. I took my small high intensity lamp and placed all these samples under it and went off to do other things. When I returned a couple of hours later, to my immense satisfaction, the surface of all three samples was a bright red. At higher magnification, I could see vast numbers of tiny red granules near the surface of the outer envelope of the organism and a bit further in, the numerous green chloroplasts. Several species of Euglena have mastered this colorful trick, but the most common one and the one which I had collected is Euglena rubra. Here is an image of Euglena acus and a link to show you E. rubra and you can immediately see the difference in terms of the pigmented particles.
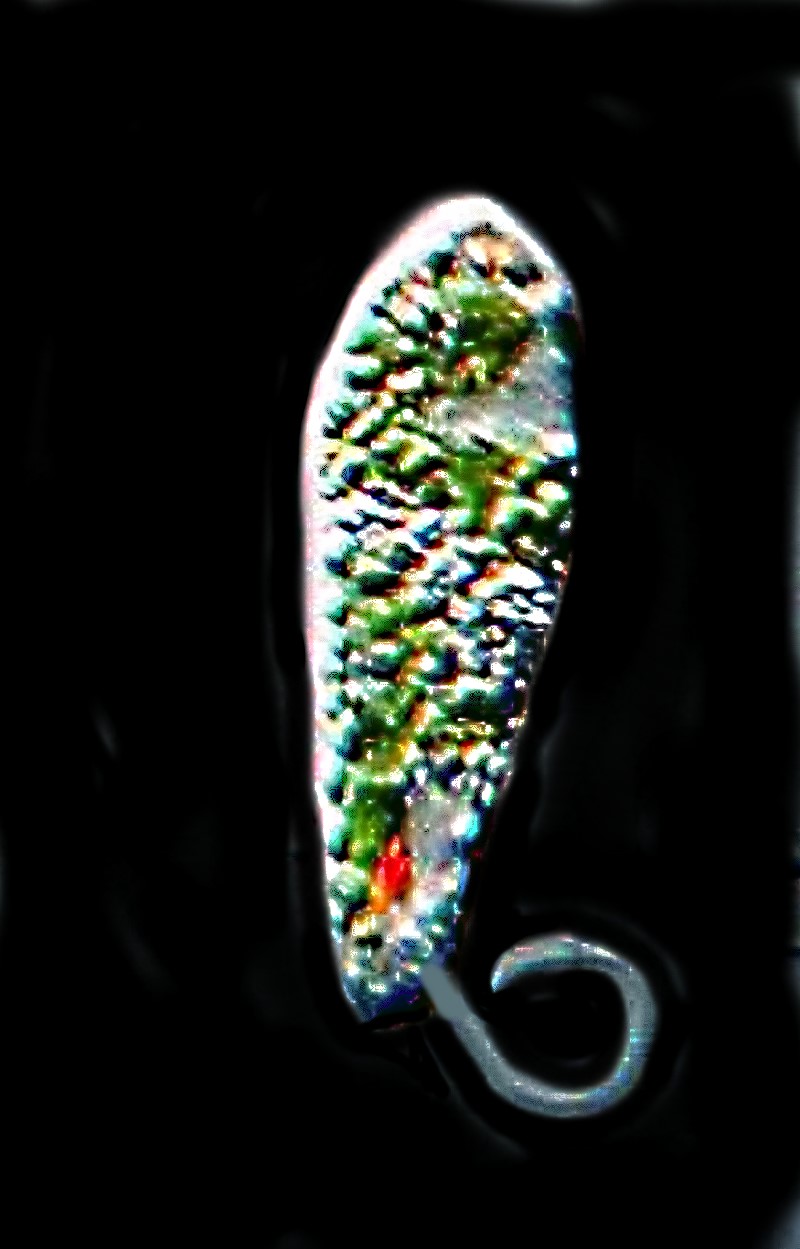
Haematococcus, which often appears in birdbaths, also has a deep red pigment as well as chloroplasts. In addition , there are the famous “red snow” algae which grow right on the surface of the snowbanks and have the distinctive odor and taste of watermelon. If you’re tempted to sample it, just taste a bit and spit it out; it can be toxic.
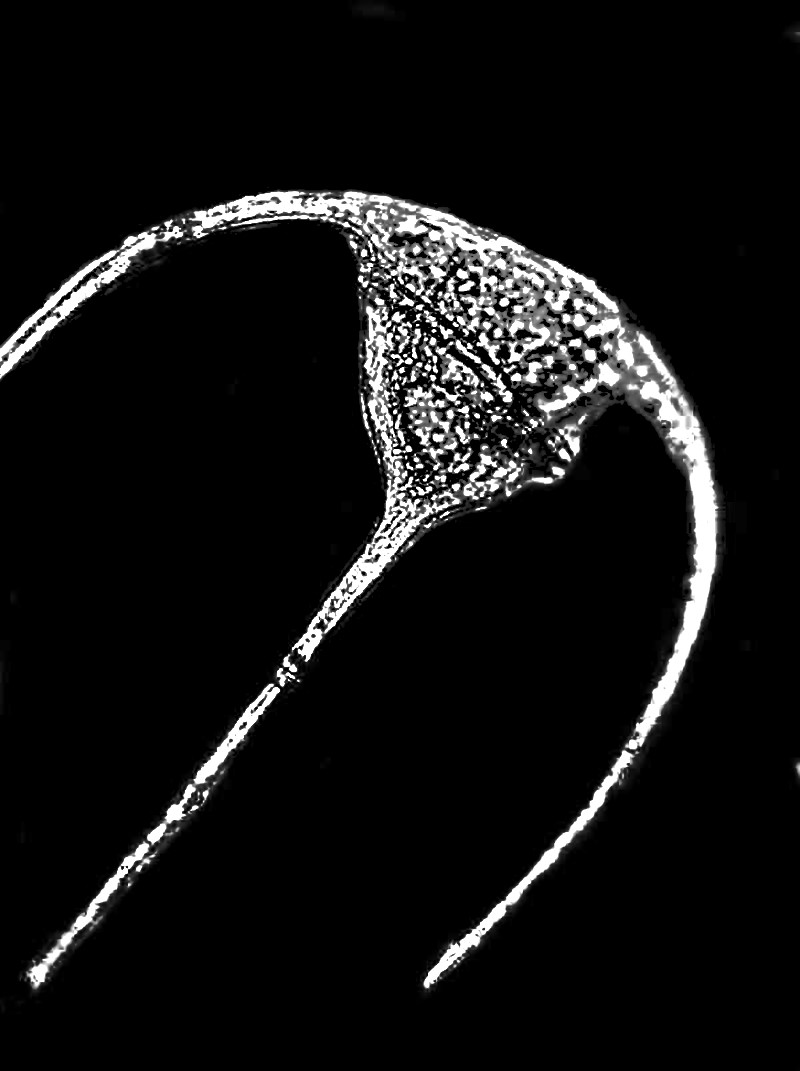
Browns, red, and yellowish-browns can be found in the dinoflagellates and almost anyone who lives near the sea has heard of, if not seen “red tide” which is the result of the explosive reproduction or so-called “blooms’ of certain species of dinoflagellates. These tiny organisms contain a toxin and it accumulates in filter feeders and mollusks and makes shellfish dangerous, even fatal, to eat during such blooms. There are also some freshwater dinoflagellates some of which, such as this specimen of Ceratium, are quite elegant.
So, there are some very colorful protists and their pigments sometimes provide helpful clues in identifying particular organisms. Alas, these pigmented beasties are a small minority and, in general, classification depends upon a series of other morphological and behavioral traits.
Another group which deserves attention with regard to locomotion is the hypotrichs (often described as “cirriates” in older texts). There is a wide variety of body design in this class, but they all possess cirri which are essentially fused bundles of cilia. Three very common genera which you are likely to come across are Oxytricha, Stylonichia, and Euplotes. The cirri function lilke minute “legs” and so one often observes hypotrichs “walking” on the substrate amid detritus. This first species is an omnivore with a preference for algae.
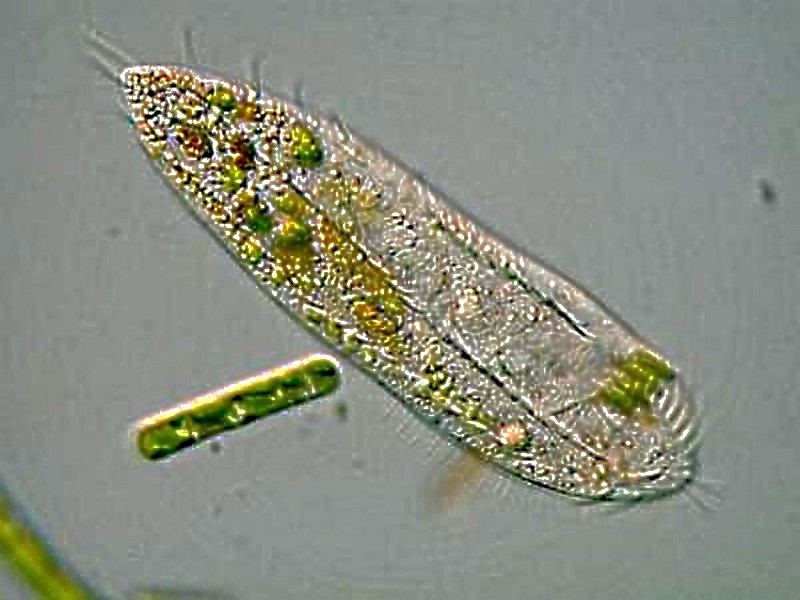
In this next image, you can clearly see the how the cilia are fused around the anterior end so that they look rather like feather tentacles.
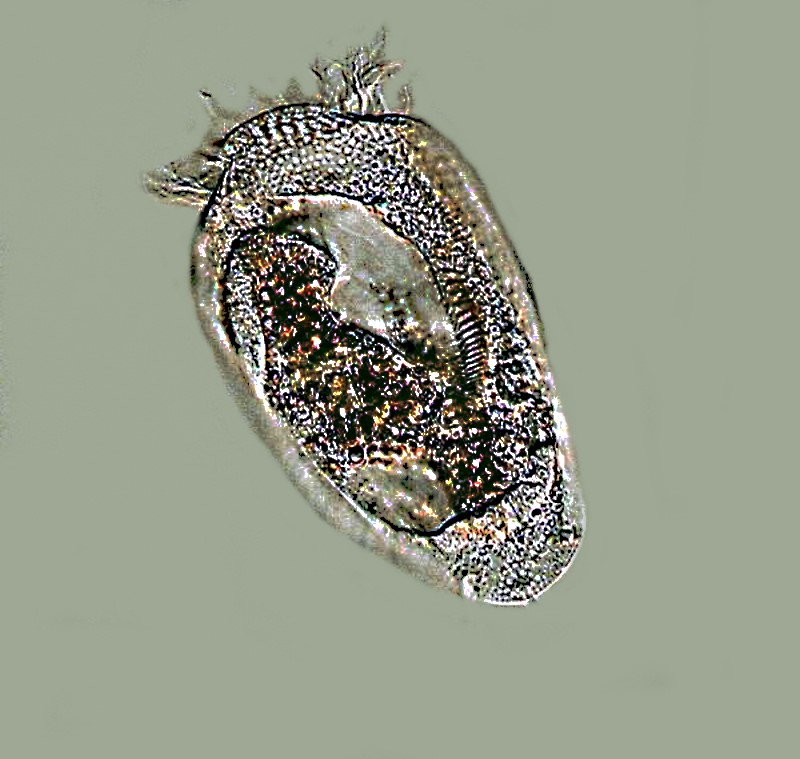
Two other morphological features that are in part related to locomotive behavior are deserving of mention: elasticity and extensility. A wide variety of protists show elasticity, including Paramecium. If you watch a Paramecium feeding in thick bacteria-rich detritus, you will notice how it bends and contorts as though its pellicle or membranous envelope were made of rubber. Euglenoids are also highly flexible as well as other flagellates, such as, Peranema acus.
One of the most spectacular displays of extensility is be found in Lacrymaria olor which can extend its “neck” up to 10 times its body length! Here is a remarkable video of this incredible organism.
Other protists have devised protective strategies that don’t involve building “houses”, rather they are armor-plated. Dinoflagellates, for example, have a series of intricate plates varying in size and shape. A common armored ciliate is Coleps which is barrel-shaped, and in most species, has posterior spines.
The structure of the mouth or cytostome is often helpful in identifying an organism. Some like Blepharisma, have a distinctive oral membrane, while other have tube-like structures leading to the oral opening and these tubes are often supported by a series of distinct rods called trichites which in some instances are used to widen or narrow the size of the opening. Of course, many protists, such as, diatoms and desmids, amoebae, and many flagellates have no distinct cytostome.
The size, shape, and nature of the macronucleus can also be of crucial importance in the process of identification and there is also the issue of micronuclei. Typically, many beginners tend to think of macronuclei as ovate or bean-shaped using observations of Paramecia as a model. In truth, there is a considerable variability in macronuclei, micronuclei and reproductive strategies. For this discussion, we shall consider primarily the macronuclei. In Stentor, Blepharisma, and Spirostomum, we find a long beaded chain nucleus, but ONLY in some species! So, this information can be helpful in trying to determine a species PROVIDED you haven’t caught the organism in one of its reproductive phases, in which case, both the macronuclei and micronuclei may be preparing all kinds of complex and morphologically bizarre transformations. To get a clear sense of the nucleatic material in protists, it is frequently necessary to stain them and, in some cases, complex procedures and reagents are required. However, in many instances, the simple technique of using Methyl Green Acetic, which when applied to living protists, will clearly demonstrate the nuclei.
One could go on and on about other aspects such as the contractile vacuole (or water expelling vesicle), the ctyopyge (or anal pore), symbiotic algae, trichocysts, toxicysts, etc. What we are becoming increasingly aware of is the fact that taxonomic systems are so complex, precisely because the organisms themselves are so complex. At this point, rather than rambling on about more and more morphological and behavioral features, I will try to draw up a sort of checksheet which some of you may find helpful in the quest to identify protists. This will only be a rough and general model and by no means pretends to be comprehensive or complete. Its central focus will be protozoa (exclusive of the Sporozoa), but sometimes discovering that a particular type of protist is not a protozoan is in itself helpful.
The first step toward identification is to make drawings from live specimens if at all possible. If the organisms are moving too fast, try to slow them down by using a drop of methyl cellulose or some other nontoxic viscous fluid or, as suggested previously, place a small tangle of cotton fibers on the slide. Sometimes, waiting until enough water under the cover glass evaporates to exert sufficient pressure will slow or stop the organism to allow close examination. One has to remember, however, that this pressure will also gradually distort the organisms and eventually result in their rupture and death. One can, from time to time, add a small drop of water at the edge of the cover glass and prolong the observation of them.
If none of these methods are sufficient, then you may want to try a chemical means of narcotizing, anesthetizing or incapacitating the organism. This can be something as simple as getting your critters “drunk” with a bit of a dilute solution of alcohol (start with a 10% solution and then work up or down as necessary). And no, you don’t need to buy a bottle of Bombay Sapphire Gin to satisfy their refined tastes. Isopropanol (rubbing alcohol) or methanol (wood alcohol) will do quite well. Some protists, like some people, clearly have the gene that makes them responsive to alcohol.
Other more complex methods involve using dilute solutions of chemicals to temporarily (or, in some instances, permanently) render the organism partly or completely inactive. I have already mentioned nickel sulfate and potassium iodide and some have bubbled cigarette smoke into a culture. (This will allow you to see firsthand that secondhand smoke can be a hazard.) Some years ago, a colleague of mine related a particularly ingenious technique using a local anesthetic called dibucaine hydrochloride. As I remember the experiment model was Tetrahymena. The anesthetic strips the cilia off the organism rendering it incapable of movement but, on transferring it back into a suitable growth medium, the cilia are regenerated and the organisms apparently come through the ordeal with no discernible damage. Whatever techniques you use, keep updating your drawings and/or making new ones. Also keep careful notes of your observations. Here are some of the things your checklist should include, if relevant or feasible.
1) Does it demonstrate the ability to move?
a) Remember some protists don’t demonstrate any form of locomotion.
b) Remember that motion does not mean that a particular protist is a protozoan. Diatoms, desmids and cyanobacteria all demonstrate movement.
2) Describe the movement. Can you determine the means of locomotion? Do you see cilia, flagella (undulipodia), or observe distinctive amoeboid motion?
3) What is the approximate size of the organism? If possible try to estimate both the length and the width. If you are using a 10x objective and 10x ocular, assuming that your microscope is not using a magnification changer nor widefield optics, then your field of view is about 1,600 microns. So, if the length of the organism is about 1/3 of the field of view, it is approximately 480 to 530 microns. With a 40x objective, your field of view is about 400 microns. If you have a micrometer, then you can measure more accurately.
4) Is the organism pigmented?
5) Is it an individual or a colony?
6) Does it have chloroplasts, chromoplasts, or pigmented particles?
7) Does it have a distinct cytostome? If so, then it’s very likely a ciliate.
8) Does the cytostome have a membrane?
9) Does the organism have a red eye-spot? If so then, it is almost certainly a phytoflagellate or a higher invertebrate, such as, Cyclops.
10) Describe the macronucleus. (Also make a drawing showing as much of its detail as possible.) Does it have more than one macronucleus? If so, how many?
11) Does it have visible micronuclei? If so, how many?
12) Does it have contractile vacuoles? If so, how many and where are they located in the organism? For example Spirostomum typically has one elongated contractile vacuole toward the posterior end, whereas Paramecium typically has two with canals leading to the central circle giving the vacuoles a flower-like appearance.
13) Does the organism have spines or plates?
14) Does it have a “house”, shell, test, lorica, membranous tube? Such a feature is frequently quite helpful in narrowing down the classification of protists.
15) Are there any unusual features in or on the external membranes of the organism? For example, In Paramecium, just under the surface of its membranous envelope are an enormous number of tiny capsules containing a minute “thread” which explodes violently when the organism is attacked by a prey or a toxic substance. A drop of dilute tannic acid will demonstrate this phenomenon, which happens so fast that you can only see the resultant “threads” which are expelled with sufficient force that some of them may be a surprising distance from the Paramecium. In a living specimen which is sufficiently compressed so that you can observe it closely, you will be able to see these tiny “capsules” all along the edge of the membrane.
16) Does the organism have any distinctly unusual internal features? Are there unusual granules, or vacuoles or special structures, such as, the paramylum bodies in which euglenoids store a starch-like carbohydrate? It is when we start investigating cytological features of the organism, that various staining techniques become invaluable. A simple case is the demonstration of the “Neutral Red” bodies in Paramecium, so named because they are visible only when stained with the “Neutral Red” stain.
There are almost certainly other significant aspects which I have overlooked in my checksheet, but you can add them to your own list as they occur to you. Also the general process can be extended. For example, you can make your own checklists which focus on amoeboid organisms, another for flagellates, and a third for ciliates. As you become more familiar with the morphological characteristics of various groups, you will find the more technical taxonomic discussions in classic texts more accessible and more helpful. The more specific and detailed you make your drawing and descriptions, the more amazed you will become at the enormous variation and fascinating diversity in the morphology of protists.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the January 2017 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .