Viewing through the eyepiece with the naked eye, or with the aid of a graticule or our camera's focussing screen, we try many times to register, as a drawing or photo, one of our interesting microscopic subjects.
If it is alive it is a frustrating sensation to view the critter’s very rapid movement across the field of view, giving us a glimpse of its delicate structure for so short an instant that we can’t fix on the image we want to show or understand.
Of course we immediately think to stop this foolish race. We remember those articles from Richard Howey (1, 2 ) that present a very useful summary of the more accessible anesthetics. We would like to try some of them…. if we have them at hand of course.
That was my situation while a beautiful Aeolosoma (footnote 1) eluded my best efforts to make some decent and well-resolved photos of its structure. Then I thought of applying an idea that came to me one of those days while web surfing.On two different occasions I saw an unusual application of one old pal: Formalin.
Commercial Formaldehyde solution, familiar to naturalists as Formalin, is a well known fixative used at 5:100 (5%) or 10:100 (10%) aqueous dilutions. But try to add a drop of this concentration to the Aeolosoma and you sadly finish up with an undecipherable twisted and wrinkled opaque thread. (See safety footnote.)But two parasitologists (3, 4) propose the use of a very dilute solution as a relaxant for Monogenea (a class of Turbellaria). They successfully use a solution diluted to 1:4000 (0.025%), that is 1 ml of commercial solution of formaldehyde dispersed in 4000 ml (4 liters) of water. Yes, you know that one teaspoon has 5 ml. They use only 1/5th of a teaspoon in 4 lt. of water!!
I don’t know when and who was the first to propose that technique, but two independent professional references (one English, one Brazilian) were enough for me. It was tempting to try this diluted solution as a relaxant for other invertebrates. I decided to try a series of dilutions with my Aeolosoma as well as other inhabitants of my aquarium. As I expect some of you may want to extend this experiment, trying the method for as many invertebrates as possible, I will describe an easy method to make the needed dilutions. (Footnote 2).
I acquired from around the home: one 2-ml hypodermic syringe, one 20 ml graduated cup, for measuring expectorant syrup; (1 hypodermic syringe of 10 or 20 ml, can do the same job, perhaps better), one 0.5 liter measuring jar from the kitchen, one large bottle of 2 liter, a medium sized funnel, and a few small flasks to receive the solutions. (Footnote 3).With the 0.5-lt. jar I measured carefully 2 lt. of plain water into the 2-lt. bottle. I adhere to the bottle one piece of adhesive tape with the edge marking the water level, and empty the bottle.
I measured with care 1 ml of formaldehyde commercial solution into the syringe and pour it into the marked bottle. Now I replenish the bottle with demineralized water (obtainable from supermarkets for car batteries) at the 2 lt. mark.
This was my Mother Dilution (MD) at the 1:2000 strength.
Now, the much anticipated experiment. I take one drop of my culture of Aeolosoma, mix one drop of MD, put a cover slide on, and run to the eyepiece.
Of course as I diluted 1:1 the MD, my animals are now in the expected dilution of 1:4000. A great disappointment; all of them are wrinkling, mostly dead, certainly not in the extended and peaceful attitude I expected. I decided to try a more diluted solution.
I take a 20 ml (4-tsp.) measure that came as a gift with the expectorant syrup bought for my grandson. It has 5 ml (1-tsp.) marks. So I measured 10 ml of the MD and put it in one of the 1-oz flasks. After rinsing the cup well, I add 10-ml of water. My dilution is as you suspect 1:4000. To extend the experiment I made three additional dilutions.
I now diluted 5 ml of MD with 10 ml of water. As I diluted the MD 3 times (from 5 to 15 ml) I now have a 2000 x 3 dilution or 1:6000.
But I wanted to use another two dilutions 1:3000 and 1:5000.
For the first I take 10 ml of MD and add only 5 ml of water. I have a dilution of 1.5 strength (2000 x 1.5 = 3000) and, for the second, 10 ml were diluted to 25 ml (2000 x 2.5 = 5000).
In summary after I have labeled and capped my flasks I finish with a series of working dilutions, 1:2000 (my MD), 3000, 4000, 5000 and 6000 aligned on my desktop. When mixed one to one drop sample:relaxant my effective relaxant solutions would be 1:4000, 6000, 8000, 10000, 12000.
I successively prepared 2 slides for each dilution. (Three or more would be better, because the drops could not be exactly equal or the animals could not react the same way due to some physiological difference, footnote 4). But I was impatient and guilty of a serious sin: I reduced my safety precautions.Fortunately on this occasion there were no consequences. The results were so consistent that I dispensed with additional experiments.
The 1:4000 dilution was not useful at all. The 1:6000 slides show some dead animals and some sluggish ones but with a much anomalous behavior, and certainly not anesthetized.
But the 1:8000 and 1:10000 slides show a consistent behavior. The animals swim more or less “naturally” but slowly. The speed was reduced over time and in 20-25 minutes many of the animals were arrested. They were alive, and all their organs appeared to function as usual. From 10 or 15 minutes on, there were some animals that allowed a search with high powers, and of course photography. I was able to use the 100x OI objective. This dilution was very successful.
The 1:12000 and 1:16000 slides were irregular and mostly unacceptable. The animals were sluggish, especially in the 1:12000 dilution, but never arrested, and always had unpredictable fast movements that prevented the use of high powers.
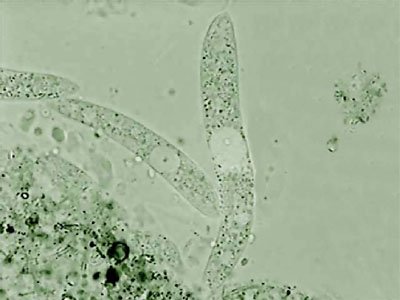
After that, I repeated the experience many times using mostly 1:8000 dilution. I was able to photograph the Aeolosoma in a lateral position, (as you can see in the photo below, top left), something which is impossible in a non relaxed animal. But what is most interesting with both successful dilutions, is the fact that most of the other micro-invertebrates present in the preparations also showed a positive response.
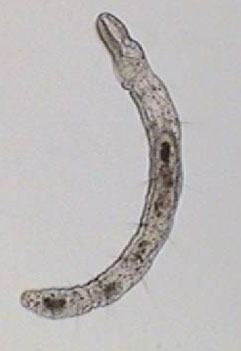
Aeolosoma - lateral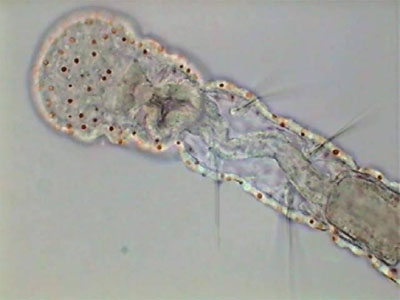
Aeolosoma - headA short list that includes only the more abundant and visible animals is: Pristina (one oligochaete of the order Naidida), and Euplotes, Stylonichia, Pleuronema, (protozoa) and two species of Nematoda.
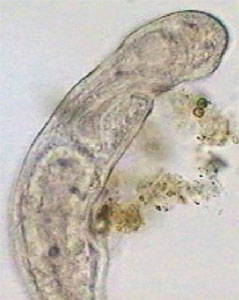
The real important thing is Pristina, because most protozoa can be stopped more effectively by fixing them. Also after a time, such as with the use of NiSO4, the contractile vacuole which expels liquids from the cell, is paralyzed by formalin which provokes the swelling of the protozoan cell and its literal explosion. Besides the two anterior images of Pristina, you can also see a more detailed photo of the gregarines, liberated by dissection of the worm.
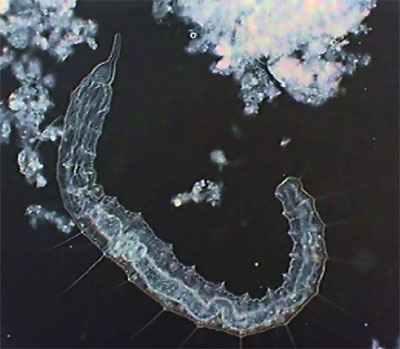
Young Pristina - lateral. Digital negative of
brightfield image to show detail.
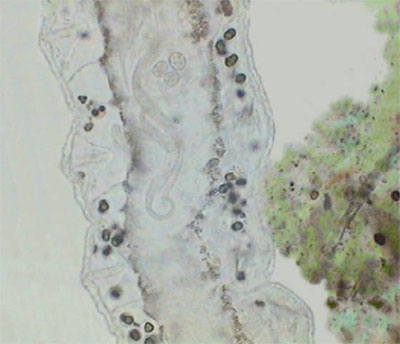
Gregarines in Pristina
(Gregarines are microscopic worm-like protozoa.)
Gregarines - releasedThe bdelloid rotifers (Philodina in this case) were all as usual tightly contracted. But other rotifers such as Colurella and Euchlanis were slowed down enough to permit some acceptable photographs.
One special case was Stenostomum, a small and agile turbellaria. Freshwater turbellaria are always difficult to anesthetize. They do not depend on muscles for their translation, but on epidermal cilia. Cilia do not respond to the same anesthetics as is the case with the two normal targets of anesthetics; the muscle or nervous system. The Stenostomum in my slides contracted a little and became somewhat sluggish. But never arrested. One or two gave me the opportunity to take some pictures, and I am really satisfied because before I could never do this. But after a few moments the animals stopped suddenly…and burst. Of them only dispersed cells remains.
Stenostomum (Catenulida) - lateralFor me the most interesting thing in addition to the anesthesia of Aeolosoma and Pristina was Vorticella relaxation. Vorticella is a very difficult animal. It has a long peduncle with a retractile myofibril and a complex belt of cilia crowns its “body”. I never had much success trying to anesthetize Vorticella and very few protozoologists claim success, mainly after long periods of soft induction with menthol, or MgCl2, or using heavy narcotics, and so on. The worst thing is that the peduncle is sensitive to narcotics (cocaine hydrochloride was most used) and the cilia is sensitive to hydrogen peroxide (H2O2). So protozoologists propose to anesthetize both parts separately. Most of the time, when the anesthesia of the peduncle is near completion, the zooid (the “body”) detaches itself from the myofibril and flies away.
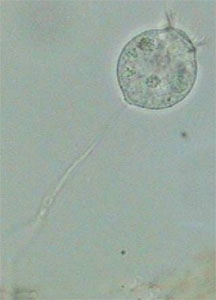
Anesthetized VorticellaI couldn’t follow the course of the anesthesia with diluted formalin. But the few specimens that I encountered searching the slides as I controlled the larger invertebrates, were all in a perfect state of integrity…and relaxation, with the ciliary crown well expanded and a naturally extended peduncle. Ignoring the ciliary movement detention, they look alive.
In summary: Formalin in very diluted concentrations is an effective relaxant for many freshwater invertebrates. For Aphanoneura and Naididae, and many protozoa a 1:8000 or 1:12000 dilution works well. This is the first time that I've seen an immediate and successful paralysis of Vorticella. The method is worth trying on marine invertebrates, and all around the systematic tree. Formalin is easily found over the counter. It is cheap, and at anyone’s reach, amateur or professional.
All comments to the author Walter Dioni are welcomed.
Walter Dioni is a retired biologist, and ecologist, with an everlasting interest in microscopy. He has published papers on the taxonomy and ecology of freshwater microinvertebrates and on some commensal Platyhelminthes from Uruguay and Argentina. He now lives in Durango, Dgo. México.
Footnotes:
1) Aeolosoma is a genus of tiny freshwater worms for a long time included in the Class Oligochaeta of the Phylum Annelida. Now they have their own Class: Aphanoneura with only one family Aeolosomatidae. For an overview of the classification (taxonomy) of the living things you can review the Micscape Magazine articles (5,6), and the beautiful book reviewed in (7). Also you could search The Tree of Life, a serious and informative project in reference (8). Return to article.2) Anesthetics and relaxants - Even if I use the two words in the same context in this article remember that there is a conceptually important difference between them. An anesthetic (as you expect if you go to the surgery room) allows the complete recovery of the anesthetized organism. A relaxant could not do that. What microscopists and naturalists want most of the time is to relax the organisms to obtain a natural appearance…and then to kill and fix them to allow for more advanced procedures, as staining or mounting. Recovery is then of no importance. Return to article.
3) Those of you that can obtain the help of your school laboratory, or have a laboratory of your own with standard equipment, can do better changing this crude assembly for 1 volumetric pipette calibrated to 1 ml, or 1 measuring pipette (Mohr pipette) of 1 or 2 ml capacity, and one graduated cylinder of 25 ml, and other of 500 ml or 1 lt. capacity, plus a 2 liter calibrated round volumetric flask, and of course the 2 lt. flask to store the MD. Return to article.
4) Think of your culture or sample as a population: some individuals would be younger, some could be sterile, some reproducing, some parasitized, and so on. This heterogeneity could be reflected in different responses to the anesthetic. Your defense against heterogeneity is to replicate the experiment many times, and to register the more frequent behavior. Return to article.
Micscape's Safety footnote
Please take care with commercial formaldehyde solutions. Formaldehyde is both toxic and a carcinogen. Only adults should handle the solutions. A typical Material Safety Data Sheet describing the hazards and safety precautions is available on the Carolina web site (Acrobat pdf file). The very dilute formalin solutions should also be handled with the appropriate care. Return to article.BIBLIOGRAPHY1.- Howey, R.L. - A bit of basic microtechnique as applied to protists.
2.- Howey, R.L. - Natural asphyxia
3.- Upton, Steve J.- Animal Parasitology, Biology 625, LABORATORY MANUAL
4.- Pavanelli, Gilberto Cezar (coordinator).- ICTIOPARASITOLOGIA (Acrobat pdf file).
5.- David B. Richman .- KINGDOMS COME (And Kingdoms go…)
6.- David Goldstein.- Classification of living things
7.- Lynn Margulis, Karlene V. Schwartz .- Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth, 3rd Edition, January 1998, W. H. Freeman & Co. (See the review of this book by David Walker.)
8.- The Tree of Life.- a collaborative work on phylogeny, with a presentation of the characteristics of important phyla. Follow the branches at http://tolweb.org/tree/phylogeny.html