
|
The genus Pseudo-nitzschia. by Rene van Wezel, UK |
Diatoms are one of the most abundant groups of plankton and as such form the base of the feeding pyramid. There they serve as the first link in the conversion of sunlight into usable energy.
During this process they also produce the oxygen we breathe, with estimates of up to a quarter of all oxygen formed by these unicellular algae (although this number probably is a bit exaggerated). This biological importance, together with the incredibly fine sculptured silica outer skeleton, has not failed to interest microscopists since Victorian times. Indeed, the development of the microscope itself is heavily linked to their use as test specimen of the optical system.

Figure 1: Acid cleaned material from Station 2706, mainly consisting of Pseudo-nitzschia's. The long needles are girdle elements from the diatom, detached by the harsh cleaning method. The diatom in the middle is a Pleurosigma. Interference contrast, Leitz ICT 40/0.70
The genus Pseudo-nitzschia is an abundant and globally widespread group of pennate diatoms. As the name suggests, it's almost Nitzschia but with enough differences to qualify as a separate genus. Like all Nitzschia's it has the typical raphe slit on the valve, which in recent evolutionary history has been moved completely to one side of the valve. This arrangement seems to make fast movement possible in the often bottom dwelling Nitzschia's, although this characteristic seems wasted on a diatom like Pseudo-nitzschia that is tight up to the movement of the water.
In marine samples, the typical stepped-chains of diatoms can easily be spotted. This formation starts after asexual reproduction when mother and daughter cells 'loosen up'. They simply slide along each other until somehow glued together at their polar ends, thereby forming the typical stepped chain pattern.
When the cell-ends can be seen separately, it is clear that you're looking at the side of the diatom. This is known as the girdle view (figure 2).


Figure 2: Pseudo-nitzschia subcurvata (?)(a) and P. australis (b), the latter in synchronous asexual reproduction. Colour phase contrast, Wild 20/0.60 phase fluotar.
The diatoms are very well preserved with Lugol's iodine solution, the internal structures can be studied in great detail (figure 3). The yellow coloured plates visible on the side of the cells are the chloroplasts, here laying along the girdle, on the same side as the raphe (not clearly visible). The nucleus is located in the middle of the cells and about ¾ of the cell's width. It is surrounded by a layer of cytoplasm, which becomes very dense near the nucleus. Within the nucleus a smaller body (the nucleolus) can sometimes be seen, this is involved in the production of the machinery needed for protein production (the ribosomes).
The striae can just about be made visible, which is amazing considering the closeness of the refractive index of silica to the mountant used (glycerine jelly).

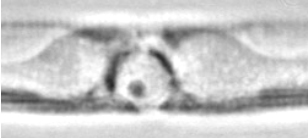

Figure 3: A chain of Pseudo-nitzschia australis in valve view, nicely preserved with Lugol's Iodine. The middle cell is shown with higher magnification. 3a: Colour phase contrast, Wild 20/0.60 phase fluotar. 3b: Phase contrast, Leitz 100/1.32 phase fluotar. 3c: Interference contrast, Leitz ICT100/1.32 fluotar.
Unfortunately, the fine silica details of the diatom are difficult to see in live material, because 1) they are obscured by the cell contents, and 2) the silica is transparent and colourless, with a refractive index close to that of water. Therefore, samples are generally cleaned from all organic content with aggressive chemicals, and then mounted on a slide with special high refractive resins that maximize contrast of the silica (in this respect, the much used canada balsam is not ideal as its refractive index of 1.51 is too close to that of the opaline silica which is 1.43).

Figure 4: Pseudo-nitzschia australis. Acid cleaned valve. Interference contrast, Leitz ICT40/0.70 fluotar

Figure 5: Pseudo-nitzschia multi-series. Gently acid cleaned, the two valves `folded open' with the connecting girdle in the middle.
Some of the fine structure on the valve that divides two species is too small to see with the light microscope. Generally, distinction is made between 'thin' (<3 µm) and wider species. For thorough classification however, the striae and fibulae spacing has to be measured, the existence of a central interspace in the raphe and the cell ends noted. Sometimes pores can be seen (P.pungens), but generally they can only be detected with an electron microscope.
Even the structure within the pores (the hymen: the silica mesh covering the base of the pore) can be typical for a certain species, although unfortunately for wild samples (non-cultured material) these structures are often too fragile to survive the harsh cleaning method.
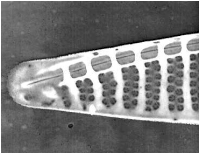
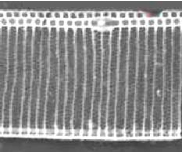
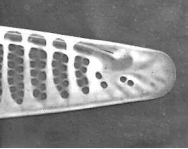
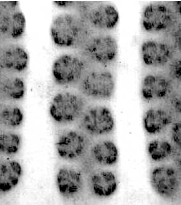
Figure 6. Pseudo-nitzschia fraudulenta. TEM micrographs showing diatom ends and middle structure (not to scale, valve width in the middle ca 6 µm). This species has a nodule in the central larger interspace, which can be seen on light microscopical level. The pores from P. fraudulenta have a more complex nature (far right) than those from P. australis (below).
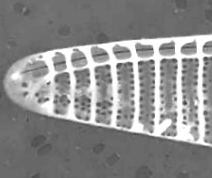
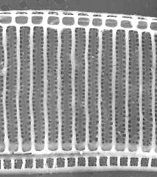
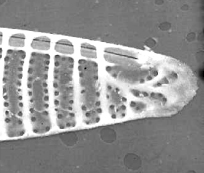
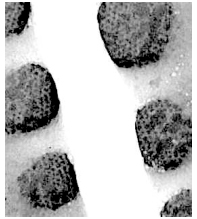
Figure 7. Pseudo-nitzschia australis. TEM micrographs showing diatom ends and middle structure (not to scale, valve width in the middle ca 7 µm). This species does have continuous raphe system. This valve still has a girdle element connected at the middle (bottom). The pores from P. australis have a simple mesh nature (far right).
Diatoms tend to 'bloom' when nutrients become available and especially coastal areas are prone to the sudden outburst of diatom growth in spring and sutumn. Although this might seem obnoxious but harmless, it easily creates chaos. For example, the large spines on some diatom species can irritate gills from fish swimming in a bloom, and the eventual death of a dense bloom consumes so much oxygen during decay that life in that zone is severely affected.
Sometimes, algae produce compounds that have a toxic effect. Pseudo-nitzschia is such a typical example where the organism can produce domoic acid (DA) under certain conditions.
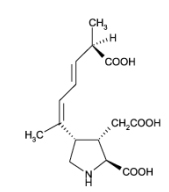 Domoic acid (figure 8, right) is a potent neurotoxin for mammals but has no obvious function for the diatom. It might be a waste product for the diatom and has no direct effects on mussels and other shellfish feeding on the DA containing diatoms. They do not seem affected at all by the chemical. Humans however (and other animal life feeding on the contaminated shellfish) can be severely affected by a syndrome called Amnesic Shellfish Poisoning (ASP). Basically, the toxin acts as a false key for the glutamate receptors in the brain, and the result can be dramatic. With the right amount of toxin, the short term memory region can be shut down completely and permanently, but without affecting the long term memory. Patients are living like in a state of clever dementia.
Domoic acid (figure 8, right) is a potent neurotoxin for mammals but has no obvious function for the diatom. It might be a waste product for the diatom and has no direct effects on mussels and other shellfish feeding on the DA containing diatoms. They do not seem affected at all by the chemical. Humans however (and other animal life feeding on the contaminated shellfish) can be severely affected by a syndrome called Amnesic Shellfish Poisoning (ASP). Basically, the toxin acts as a false key for the glutamate receptors in the brain, and the result can be dramatic. With the right amount of toxin, the short term memory region can be shut down completely and permanently, but without affecting the long term memory. Patients are living like in a state of clever dementia.
Because of this indirect link between the toxic algae and the actually poisoned organism (us), the source of DA induced poisoning was only rather recently discovered as being a bloom of P.pungens for the coast of Canada in 1987.
Coastal areas are naturally prone to bloom formation, as natural upwelling of nutrients along the coastline produce favourable conditions for growth. But also human-induced enrichment like sewage, runoff from agricultural land and, not in the least, fish farming do contribute dramatically to the chance of the occurrence of a Harmful Algal Bloom.
Technicalities:
The samples were derived from the SW coast of Ireland during a cruise on board the Celtic Voyager, 18-31 August 1998. During this time, the main diatom flora was Pseudo-nitzschia australis.
Vertical net hauls were preserved with Lugol's iodine solution and permanent mounts were made in glycerine jelly, or acid cleaned for diatom analysis.
I am indebted to the crew of the Celtic Voyager and the Marine Microbiology section of the Martin Ryan Institute in Galway, Ireland, where I had the opportunity to study the plankton flora around Ireland.
All comments to the author Rene van Wezel are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the
on-line monthly magazine of the Microscopy UK
web
site at
Microscopy-UK