|
|
A Gallery of Salicylic Acid Photomicrographs (using a variety of illumination
techniques) |
|
|
A Gallery of Salicylic Acid Photomicrographs (using a variety of illumination
techniques) |
Salicylic
acid and its chemical derivatives have long been useful in the medical
drug arsenal. Birch bark and wintergreen leaves both contain
salicylic acid esters that were valuable in an earlier age as medicines
for pain relief and as antiseptics. The acid itself is still used
today in ointments for skin diseases.
If salicylic acid is reacted with
methanol (wood alcohol), the product formed is methyl salicylate,
commonly known as “oil of wintergreen”.
(The preparation of this ester is a common lab in secondary school
organic chemistry.) The aromatic product is a common flavouring,
and is also used in ointments for sore muscles (giving them the
distinctive “wintergreen” smell as an additional benefit).
When salicylic acid is reacted with
acetic acid, (from which vinegar is produced), the product is the most
widely used synthetically produced drug in the world, acetylsalicylic
acid (ASA), commonly called “aspirin”.
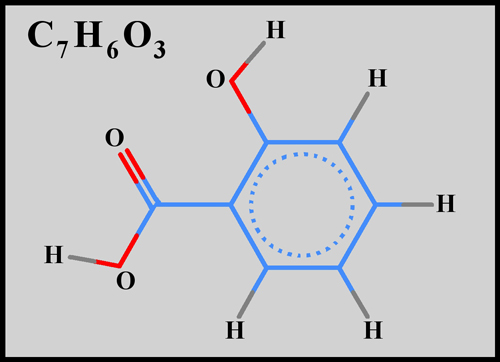
Salicylic acid itself is a benzene
ring with alcohol (-OH), and carboxylic acid (-COOH) functional groups
replacing two adjacent hydrogens. The structural formula and
molecular shape can be seen below. (The illustrations were
produced using HyperChem.)
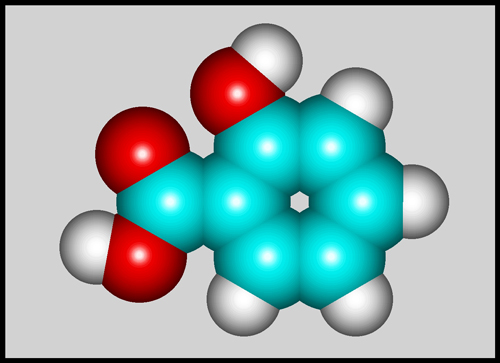
For comparison, here is the
molecular shape of aspirin, its most important derivative.
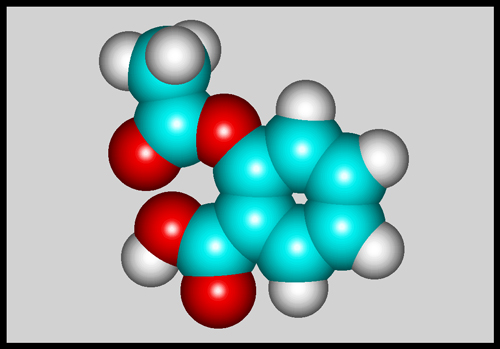
Salicylic
acid is usually provided as white, powdery crystals, which have a
melting temperature of 161 degrees Celsius. The relatively low
melting point means that a melt specimen can easily be prepared by
heating with an alcohol lamp, a small quantity sandwiched between slide
and coverglass.
Note that the MSDS safely
information for the compound warns that the solid is harmful by
inhalation, ingestion and skin absorption. Care should therefore
be taken when handling the crystals.
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using several
illumination techniques: phase contrast, dark-ground, and polarized
light. Crossed polars were used in all polarized light
images. Compensators, ( lambda and lambda/4 plates ), were
utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x
or 25x flat-field objective formed the original image and a 10x
Periplan eyepiece projected the image to the camera lens.
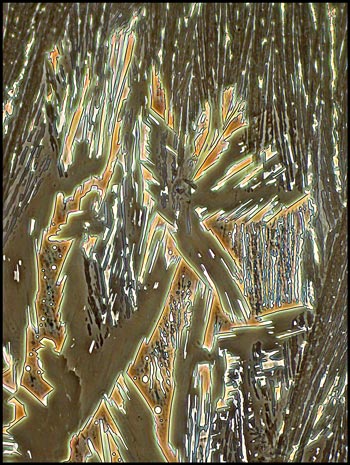
Phase
Contrast Illumination
In an earlier
article, I showed the results obtained by employing phase contrast
illumination to investigate crystal melt specimens. As expected,
most chemicals gave disappointing results. A few however proved
the exception to the rule, and of these, salicylic acid proved to be
the most photogenic. Phase contrast works best on biological
materials because they have the required prerequisites, parts with
differences in refractive index and thickness. One would expect
that a melt specimen using pure compound would have a constant
refractive index, and would therefore show only a slight contrast
difference due to subtle changes in the thickness of the trapped
crystals. What is surprising is that many images show some
colour, probably due to interference phenomena.
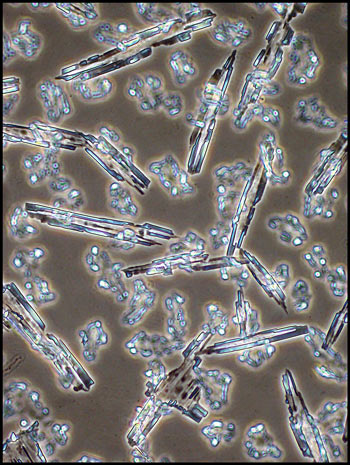
The first image in the article, and
the three below give an indication of the striking architectural
structures that this method reveals.

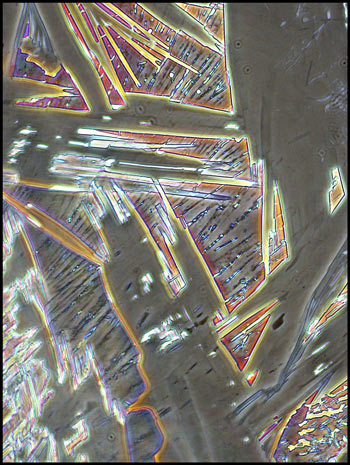
Many of the larger structures are
surrounded by a granular looking matrix.
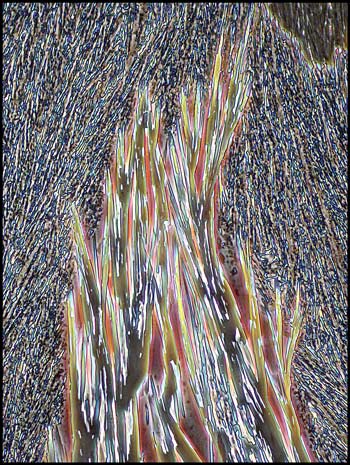

Notice the distinctive pink and
blue colours in the two images below. One might guess that these
are produced by crossed polarizers, but they are not!

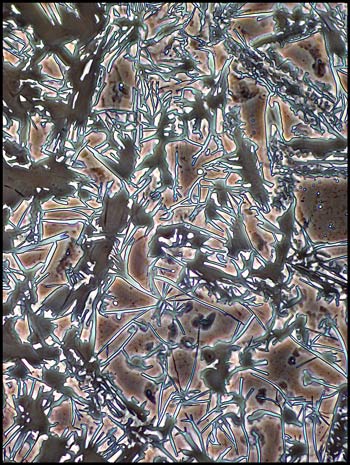
I experimented by ringing each
coverglass with a small bead of fingernail polish. Since this
acts as a solvent for the salicylic acid, a one to two millimetre wide
ring of the crystals dissolved. As the solvent evaporated, some
of the acid recrystallized forming the strange amoeba like structures
that can be seen in the following two images.

In some locations away from the coverglass edge, the smooth gray-brown
of the phase contrast field seemed to contain small individual
crystals. These can be seen in the image on the right.

Polarized
Light Illumination
The next section of the article
demonstrates the results of using crossed polars and a combination of
two lambda/4 compensators, or a combination of lambda/4 and lambda
compensators to illuminate the specimen.
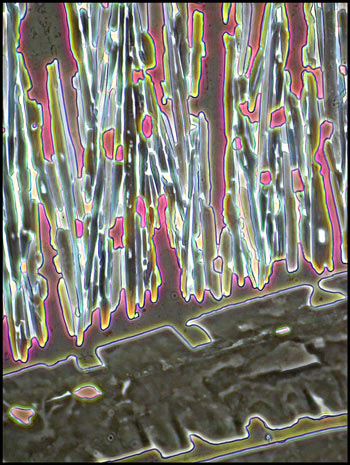
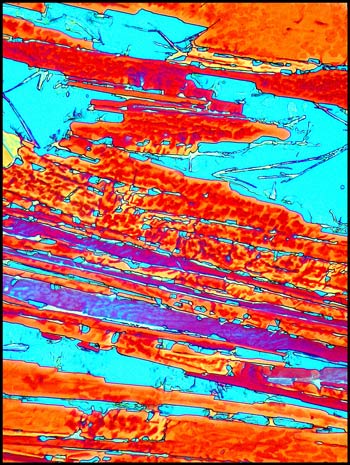
In many areas, large randomly
oriented needle-like structures formed, like the ones in the image
below.

The first image on the top left
shows a typical field. The other three images (from the same
field) were taken using higher magnification objectives.




The blue background in the
following photomicrographs was produced with the lambda/4 + lambda
combination. The stage of a polarizing microscope rotates, and an
angle was chosen which gave this particular hue.

These
unusual crystal structures formed only in one very small location on a
slide.

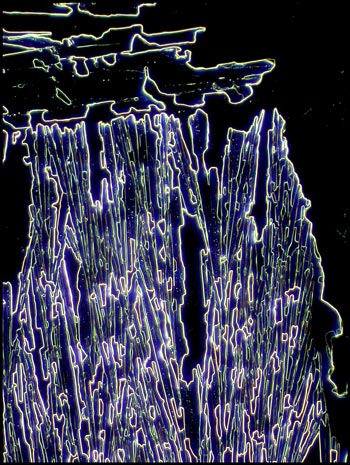
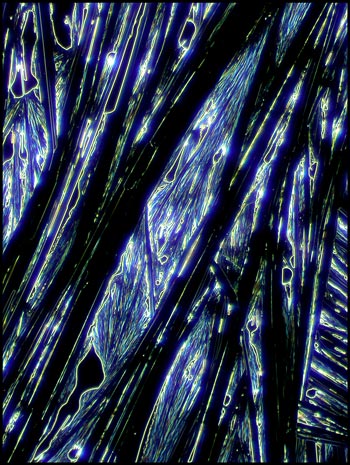
Dark-ground
Illumination
Instead of using a dark-ground
condenser in this instance, I chose to use one of the phase contrast
condenser “phase” settings which produced a black background with a
non-phase objective. This accounts for the slight colouration in
the images.

For this chemical, I prefer the
phase contrast images to those produced by the other methods.
It’s unfortunate that this type of illumination works with so few
compounds!
Published in the July
2005 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .