
|
How To Milk A Weed: Looking At Crystals In Plant Juices: Part I by Richard L. Howey, Wyoming, USA |
When I was a boy growing up in the Central Plains in Eastern Nebraska, milkweed was common and it was a pleasant entertainment to collect a few dry pods, pop them completely open and blow the remaining white, fluffy seeds into the air. I knew that it got its name from the milky sap in the leaves and stems, but it never occurred to me to look at any of it under the microscope. After all, what could liquid plant juices offer that would be of interest? The answer to that question, as I have since discovered, is—quite a lot. As it turns out, a fairly wide range of plants have fluids that contain minute crystals and they can be in the leaves or the stems or the fleshy seed pods or the roots.
To get the maximum enjoyment out of this investigation, you will need a polarizer and analyzer for your microscope. If you have compensators as well, so much the better. If you don’t, you can make some of your own by using thin sheets of mica, thin pieces of certain types of plastic (such as those you can cut from Petri dishes), or even bits of cellophane or cellophane tape attached to a thin disk.
Where to begin? If you happen to have rhubarb growing in your yard, that’s a good place to start. If not, you may well find some at your local grocery store. However, you want to buy a stalk which still has a big green leaf attached, because that’s where you’re going to find the calcium oxalate crystals in abundance. They are essentially oxalic acid and are poisonous which is why one doesn’t eat the leaves, but only the stalks. So, go to a grocery store with only mediocre produce which is primarily interested in maximum profit. They’ll sell you rhubarb by the pound with large leaves still attached so that they can charge you more. However, in this case, you won’t mind at all and all you need is a single stalk with a nice big leaf. If the produce manager happens to see you leaving with this rhubarb stalk with a leaf the size of a small umbrella, he’ll be thinking to himself–“What a sap!” of course he’ll be right on target, because that’s what you’re after–the sap. Unfortunately, the only stalks I could find had been well-trimmed with only small vestiges of leaves. This affected results I got, but more of that later.
When you get your treasure home to your lab, slice a small section of the leaf where there is an abundance of sap, for example, across one of the veins, and smear some across a couple of clean slides. The process of crystallization will take place more quickly if you use a slide that has been slightly warmed. You can set the slide aside and examine it later after the crystals have formed or you can enhance your pleasure by watching the slide through the microscope when the evaporation has reached the point when the crystals are just beginning to form. My preference is the latter, since for me there is always something quite wonderful, almost magical, in observing geometrical forms emerge from an amorphous liquid. In any case, examine the crystals in both brightfield and polarized illumination. You may also want to try darkfield. These crystals are acicular or needle-shaped and are called raphides. However, here is where my samples ended up being different as a consequence of the sections being cut so close to the stalk and so, I got crystal formations of a rather different, but nonetheless quite pleasing, character.
However, I can show you some very nice raphides cut from the developing ovary of a Fuchsia. For a number of years now, we have put hanging baskets of fuchsias on our front and side porches for the summer which here usually is on a Tuesday in July. If, after the flower drops, you don’t trim the stem, then soon you will see the bulge of a seed pod developing. This is wonderful for the propagation of fuchsias in an appropriate climate–which ours definitely is not. Also, if you let these juicy seed pods develop, this diverts energy away from the blooms, so the avid gardener pinches off the stems of those that have already bloomed in order to get maximal flowering. However, no microscopist worth his or her salt would miss an opportunity to see the crystals in fuchsia juice.

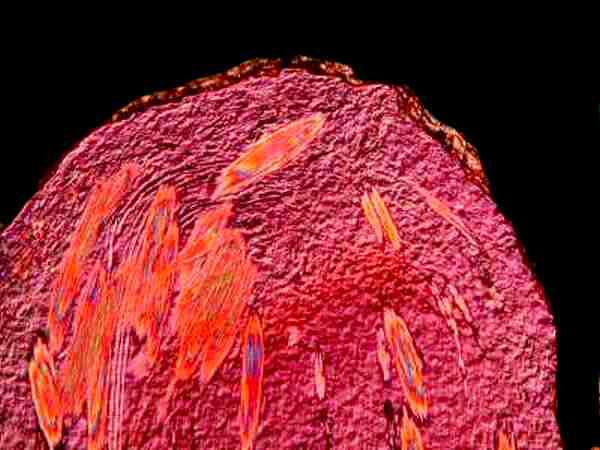
Another plant which is almost universally available, and is almost universally regarded as a nuisance by gardeners and lawnkeepers, is, of course, the dandelion. In the milky sap of its stem you will find crystals. In some parts of the world, particular varieties of dandelions have been cultivated for this milky latex.

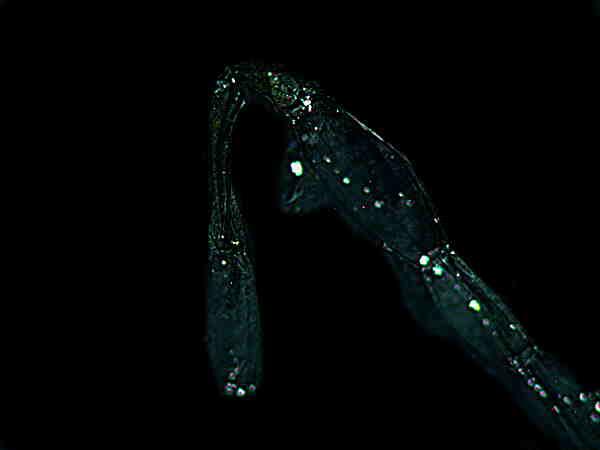

Iris often benefit from being transplanted every few years and if you trim off a bit from the root, you will find some very nice raphides. This year we didn’t transplant, so I had to settle for trying to coax a crystal or two out of a leaf.
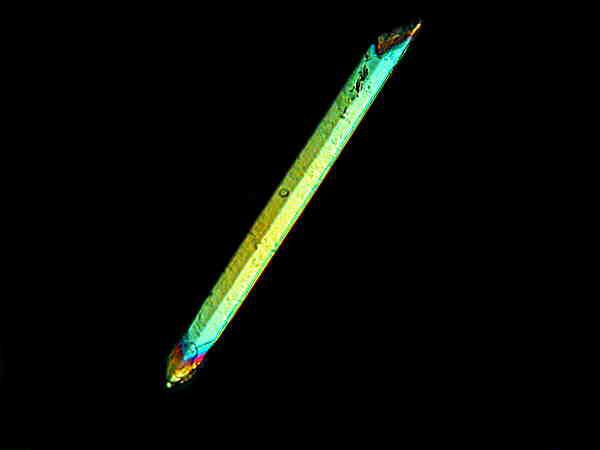
Quite a lovely thing to emerge from a tough fibrous leaf.
Not all crystals are raphides. Some are calcium carbonate, some are silica, some are circular and some are stellate or star-shaped.
As you poke around your house plants and wander through your yard and alleyways, all kinds of opportunities to investigate plant juices will present themselves. This morning, in one of our flower beds, I noticed a 6 inch specimen of one of those noxious, anti-capitalist weeds which no self-respecting American gardener would allow–a Russian thistle! I seemed to recall that in addition to its quite pretty purple flowers, it has a milky sap in the leaves and stems. I tried one and then a couple of days later tried another one and didn’t get any interesting results. So much for my memory!
If you have any cacti, euphorbia, succulents, sedums, or stoneworts around, here is an excellent opportunity to conduct some investigations that should produce some interesting crystals. Plants that have a relatively thick, syrupy juice frequently produce crystals.
The biochemistry of plants is fascinatingly complex. Just consider how many medications, foodstuffs, and textile fibers we have obtained from plants. Acetylsalicylic acid, which is the active ingredient in aspirin, can be found in willow bark; quinine is found in the bark of the cinchona tree; digitalis, a powerful heart stimulant, was originally extracted from foxglove, and the list goes on and on. Many of these substance are now synthesized, but nature provided the original clues and raw materials. Some plants have even evolved in such a way that they have produced their own insecticides. It might even be the case that rhubarb and other plants containing calcium oxalates developed these as a deterrent to plant pests. However, insects are maddeningly adaptable and there’s almost always some critter that eventually figures a way around virtually any counter-measure with which it is confronted. Our increasing knowledge of nature’s grand creation–the 4 voice fugue of DNA–is revealing to us a cosmic symphony of possibilities which outruns human imagination–possibilities both grand and deadly. Using these ultramicroscopic codes, even something as “primitive” as a virus has “learned” how to utilize these amino acids to transform itself from something relatively benign into a monstrously lethal threat to human beings and other creatures. Even plants are attacked by viruses and one which used to cause great consternation was the Tobacco Mosaic virus but, times change, and now some people would cheer for the victory of that virus.
It is not improbable that the acids, which certain plants developed and concentrated in their fruits where the seeds are, evolved as a protective measure. This is a shrewd adaptation, since it enhances the possibility for the survival of the seeds for the production of a new generation of plants. The same is likely true for the capsaicin in the various species of chili peppers. In these instances, although we have become major consumers of citrus fruits and peppers, we also cultivate them in enormous numbers and have also found ways to create strains which are resistant to pests, drought, temperature, and diseases. So, the plant can benefit and we can enhance our gustatory pleasure. However, we need always to be aware of the dangers of limiting genetic variation and relying on just a few strains of a given plant. Insects, fungi, bacteria, and viruses are all busy launching counterattacks and if they get lucky, we could lose one or more major food sources.
Anyway, all of this is a long-winded way of suggesting that these defenses which some plants devised might produce some interesting crystals for us to examine. So, take a lemon or lime, squeeze a bit of juice on the slide and you’ll get crystals, in this case, citric acid crystals. Peppers I know nothing about. My stomach has set up a block between itself and my brain after reprogramming it; it’s like one of those parental control gizmos on TVs and computers. In the grocery store, if I’m going after avocados and they happen to be next to chili peppers, my stomach starts doing volcanic eruptions. So, your assignment, should you chose to accept it, is to try the juices of various chili peppers for crystals and report back to me. Besides, why should I do all the work?
The problem with this project is that one can end up with a bewildering overstock of produce and a small botanical garden which threatens to take over the house. My wife has a special fondness for zygocacti and hoyas (or should it be zygocactas and hoyi?–humans are notoriously inconsistent). A case in point: some years ago, we were in a large nursery in Fort Collins about 70 miles south of Laramie and they had an enormous, magnificent hoya in a hanging basket. The leaves were an elongate heart-shape and were the size of the hand of a small child. We inquired as to whether or not they had a small one, but they didn’t. The problem was not only the size of this botanical creature, but also that it had a price tag of $90 and that was 25 years ago and not affordable on the salary of an associate professor. But my wife’s plant lust had possessed her and she asked the very pleasant saleswoman if it would be possible for us to buy a cutting from this huge monster. This, by now in my wife’s eyes paragon-of-horticultural virtue, smiled and said: “No problem. I’ll just give you a cutting. No charge.” A clever stratagem, since, then of course, we felt obligated to buy some other plants even though it was by no means $90 worth. After presenting us with the cutting, the woman said: “Now, you’ll have to be patient. This particular hoya grows slowly.” My wife potted it carefully and hung it in a west window in a 16 x 16 foot room which we call the music room because it has my piano in it. I’m beginning to think that the woman was teasing us, because within months, I was beginning to glance nervously to my left at the tendrils which seemed to be creeping ever close. For those of you have seen the film Little Shop of Horrors, I kept expecting one day to hear it say: “Feed me!” This botanical creature would, left to its own devices, I am sure, have taken over the entire room. Three times now, we have ventured into the music room, machetes in hand (I exaggerate only slightly), to severely “prune” (hack away at) this beast disguised as a plant. Things will go well for some months and then it starts over again. Even worse, I discovered that each time we “prune”, my wife starts a new cutting. I can see the headline now: GIANT HOYA STRANGLES HUSBAND, WIFE, TWO CATS AND THREATENS NEIGHBORS.
So, what’s the point? The point is that if you get intrigued with examining plants–whether it be crystals in the juices, cell structure, photoperiodism, whatever–beware, they can take over your house.
However, my investigations began with the yards where weeds and shrubs are taking over. I don’t mind the bushes, in fact, I encourage them unless they are of a political ilk and become Bushes. Every late spring and early summer is a battle with dandelions and thistles. So, to start this experiment, I armed myself with a pocketful of plastic sandwich bags, a sharp pocket knife, and a marker to label the bags. In addition to thistle and dandelion, I took samples of sweet pea, iris leaf, unripe chokecherries, daylily, geranium, Cheyenne privet, and Oriental poppy leaf.
I started with a poppy leaf. There is, as you know, a long tradition of a search for the juice of the poppy. The opium poppy (Papaver somniferum) has somewhat different characteristics than the ordinary garden poppy and genuine opium poppies are banned from importation and cultivation in this country. The leaves of my Oriental poppy (Papaver orientale) don’t have much in the way of “juice”; I think that’s probably mostly in the flower stalks and perhaps the pods and roots. However, ours haven’t bloomed yet this year and, if I were to whack off a stem with a bud, my wife might inflict damage on my own delicate person. However, by squeezing and pressing and slicing the leaf, I did get a few amorphous crystals of dubious interest. I’ll try again later in the season after they have finished blooming.
At this point, as frequently happens, my curiosity sidetracked me. The leaf possessed large numbers of small hairs and plant hairs and spines are an interesting subject in themselves. On the underside, along the edges and border of the veins, there are large numbers of minute spherical structures. At first, I thought these might be scales of some sort, but that turned out not to be the case. I did some scrapings from the underside of the leaf with a micro-scalpel. Examining these with a compound microscope revealed a marvelous variety of cells and structures which I have vowed to study further. My present knowledge of plant histology is appallingly small. As I was perusing the leaf with my stereo-dissecting microscope, I noticed some movement. When I zoomed in, I discovered lots of plant mites busily sucking the sap from the leaves and excreting spherical droplets.

I think that this one is a “mama” mite, because there were also a lot of smaller ones nearby busily feeding away.

I watched this larger one for several minutes and observed that inside, in the abdomen, there was a kind of pumping action that is undoubtedly associated with the feeding activity. This made me think that perhaps all of the species of poppies may have some “special” chemicals in them, because the mites appeared quite blissful. As a matter of fact, a number of species of poppies contain a variety of alkaloid compounds. Interestingly, around the mites there were also a lot of web-like single strands. Mites are, of course, related to spiders, but it never occurred to me that they might make webs but, of course, they do.
The network of poppy leaf hairs certainly seems to provide a desirable habitat for interlopers for, not only did I find mites, but a couple of small larvae

and a fly that was actively feeding

or it was until I fixed it in alcohol and transferred it to a slide to get a better idea of what it was like.

As you can see, the transfer process resulted in some damage to one of the wings.
The intriguing thing is that all of this came from a part of just one leaf. So, time permitting, I am going to undertake a much more extensive investigation of Oriental poppy plants. For the moment I’ll just give you a close-up peak at the hairs.

This is one under polarized light with the polar's fully crossed.

This shows a couple of hairs with the polar's only partially crossed. When under low magnification it looks simply like a rather ordinary plant hair, while here under higher magnification it looks like a medieval spiked lance.
But, back to crystals. Next I looked at a slide with a smear from a geranium leaf. The first thing I encountered was a nice, colorful little crystal.
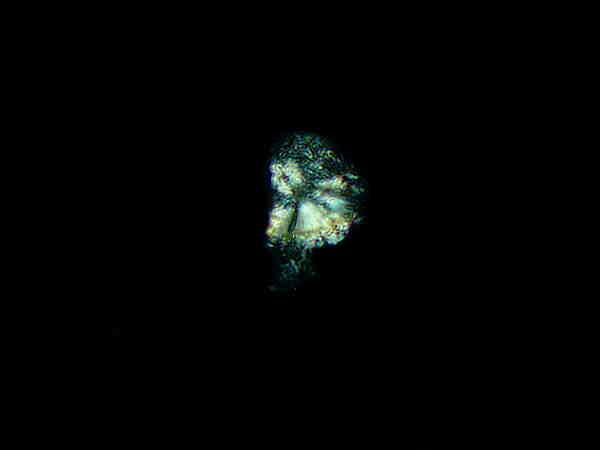
As I continued to scan the slide I came across an interesting grouping of 3 small flattened ovoid structures.

At first I thought that these were probably just some cellulose “envelopes” of cells in the leaf. I partially uncrossed the polar's to get another view and here’s what I saw.

For some reason, this second view looked to me as though these structures might be calcareous. Unfortunately, at the time, I didn’t have the wit to add a bit of dilute hydrochloric or acetic acid to see if these structure would dissolve. If they had, this would have been a good indication of their crystalline nature; if not, then they were likely cellulose compounds.
The sweet pea, the Cheyenne privet and, surprisingly, the thistle produced only extremely tiny crystals which were not interesting. I expected more from the thistle and I’ll have another go at it. This time I think I’ll take a section of root. Thistles are extremely well-armed and one must use stout gloves to handle them. I wondered what makes them so obnoxious. Under the microscope, one can quickly discover at least part of the answer. There are may minute hairs which for most of their length have a greenish-white color, but the tips, where the very, very sharp points are, have a brownish color and have become hardened. So, when you invade their territory with your fingers, those tips break off and embed themselves. I suspect they also release a mild irritant rather in the manner of the stinging nettle. It’s a clever strategy because the hair can then form a new hardened tip for its future defense.
Before we go on to the other examples which did provide some results, let me add a cautionary note. Plant juices are saps, syrups, sticky liquids and some are stickier than others, which means, you may have to let your slide smears dry for a day or two before you get any crystals. I found this out myself by being overly anxious to examine my samples. I got my first reminder when I was examining a small crystal under fully crossed polar's when suddenly new crystals began forming out of the darkness as more of the surrounding liquid evaporated. The second reminder came as I was trying to center a small crystal to photograph it and to facilitate the process I partially uncrossed the polar's which revealed a small pool of liquid surrounding the crystal. So, don’t throw out your slides after the initial examination; check them for several days and keep records as to which plants (and which parts of the plants) give good results.
The green, hard chokecherries yielded some small crystals.

I suspect that as the berries “juice up” and ripen, they may yield more. Its geometric form rather reminds me of some of the very small crystals which Magnesium chloride sometimes produces.
I cut a section from the blade of iris which, as I mentioned earlier, is tough and fibrous. The iris blade is not very juicy and I used a micro-scalpel to make a number of small incisions and then scraped and pressed the edge to get enough liquid to make a smear on a slide.
I followed a similar procedure with the daylily. One of the flowers had bloomed and withered and I cut the stem just below the ovary and it was a section of this that I minced with the micro-scalpel. Again, I didn’t expect any particularly interesting results. Here are a few examples of what I got.
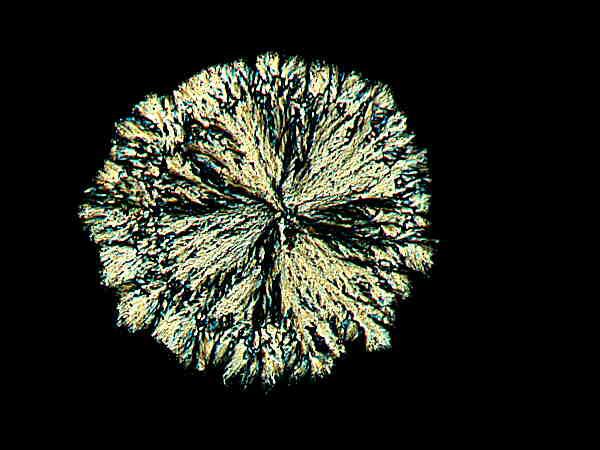

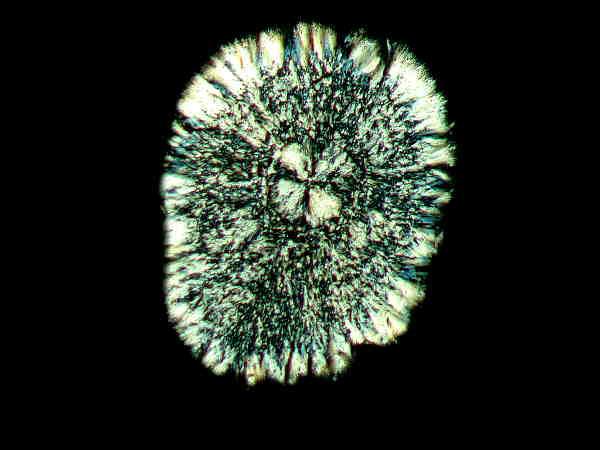
These strongly remind me of some of the disk-like crystals one sometimes encounters in Vitamin C preparations.
As usual, I’ve rambled on and this has essay gotten rather long and we’re still out in the yard and haven’t gotten to the houseplants, so I’ll talk about those next month.
In the meantime, if you find some weeds or yard plants with some interesting crystals, please let me know.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK