Observing Bacteria
Robert Pavlis, Girard, Kansas USA
Observing Living Bacteria with Phase Contrast:
Living bacteria are difficult to observe with microscopes for two fundamental reasons:
(1) Bacteria are small—dimensions typically run between 0.5 and 5 microns. Their dimensions are similar to the wavelength of visible light (about 0.38 to 0.72 microns).
(2) Most bacteria are colourless. However, their index of refraction is greater than water.
Because bacteria are so small, objectives used to observe them must have extremely good resolution and high numerical aperture. However, objects that are visible only because of refractive index differences are extremely difficult to observe with high numerical aperture objectives because of the profound loss of contrast that occurs with these objectives! Phase contrast objectives would seem to be the ideal choice.
The highest numerical aperture objectives are oil immersion objectives. These require that all of the space between the specimen being observed and the objective be filled with material having an index of refraction equal that of the lower element of the objective. This means that the specimen itself must be immersed in oil having this index of refraction. But Earth's microorganisms are water based and live in water! The index of refraction of water is about 1.33, far below the 1.515 index of immersion oil! When light passes between media having different refractive indices, it is refracted through an angle in accordance with Snell's law. This induces spherical aberration, and this becomes much worse with high numerical aperture lenses because of their high angular aperture.
Therefore, if one should place a drop of immersion oil on top of a glass cover slip over an aqueous preparation containing the bacteria and attempt to observe the result, there will be severe spherical aberration unless the bacteria being observed are nearly in contact with the cover slip of the preparation.
In reality the best method to observe living bacteria is to use WATER immersion phase contrast objectives. Unfortunately these are EXTREMELY difficult to locate, and most are also extremely expensive. It is really very odd that manufacturers do not make all of their high power phase contrast objectives for water immersion since the primary use of phase contrast objectives is to observe living organisms or tissue in aqueous media.
Nevertheless, living bacteria can be observed with oil immersion because of the simple fact that when they are close to the cover slip the degradation of the image is tolerable.
Oil immersion lenses are commonly manufactured with numerical aperture up to about 1.40, usually the higher numerical aperture ones are apochromats. With really high numerical aperture optical laws demand that there be a constant 1.51 index of refraction between the object being observed and the objective. With somewhat lower numerical apertures the problems are reduced.
Phase objectives, however typically are produced with numerical apertures no higher than 1.25 or so because the index of water is only 1.33.
It is sometimes somewhat difficult to obtain used 90X and 100X and new ones are extremely expensive. The primary reason that used ones seem in short supply is because careless microscope users in academic and industrial laboratory settings seem to have a tendency to destroy these lenses!!! Most of these lenses are spring loaded to prevent driving them through slides during focusing. Nevertheless, many of these lenses are damaged by focusing accidents. Many others meet their demise through careless cleaning. After each use the oil needs to be removed, and they can be scratched by careless cleaning. A very large fraction of the destroyed lenses get destroyed by using inappropriate solvents during the cleaning operation—solvents that work their way past the lens seals and into the inner elements of the lens.
Once one has one or more of these lenses one must remember always to take especially good care of them! The oil must be removed after each use, and they must not be cleaned with aggressive solvents. Cargille type A immersion oil is probably the best one for general use because it can be removed with saturated hydrocarbon solvents like heptane that tend not to attack other materials. (Cargille type B oil has similar solubility properties, but it is a much higher viscosity.)
The better alternative is water immersion lenses. There are two types of these—those that are corrected for having water all the way between the objective and the subject with no cover slip, and those that are corrected for having a cover slip with water above and below it. The latter design is generally preferred because they confine the organisms being studied to a relatively smaller space.
Some time ago I was fortunate enough to obtain a LOMO 1.23 na 70x apochromatic phase contrast water immersion objective. These are based on a very old zeiss design. This lens is designed for use with a cover slip, and it has a collar for correction for cover slip thickness.
This lens is also designed to be par focal with other 31mm RMS lenses. Unfortunately, however, the phase ring is not a standard size. I have never been able to find a turret phase contrast condenser that has a phase annulus to match this!
One solution to this problem would be to obtain one of the Leitz Heine condensers. They are very scarce, and consequently very expensive. Another solution would be to replace the phase rings in a standard phase contrast condenser. This would be VERY hard to accomplish.
I have developed two less expensive and difficult techniques to utilise this wonderful objective. The first involves an old Olympus CH microscope that a friend gave me. It utilises an inexpensive and somewhat crude method of dealing with the problem of having a constantly variable diameter phase condenser. Still, it works! This is a really peculiar phase condenser with the phase annulus on top of the condenser, rather than in its usual position. By moving the condenser up and down it projects a continuously variable size annulus into the objective. This system works very well with high power objectives like this, but poorly with low power ones. Here, fortunately, we are dealing with an high power objective!
Unfortunately the Olympus CH does have enough focus travel to utilise the old RMS standard objectives. What I needed was an objective extension. These are a bit hard to find. Just as I was about to machine one I discovered that a company called Rolyn optics sells 10mm extensions of this sort. I obtained one, and now I had a very good way to use this objective.
The second technique involves machining a phase ring to place under the condenser. It is particularly easy to do this with the Olympus BHA microscope. Some time ago I machined a phase ring for a Wild M40 for a 4X phase objective. Although I did a perfect job machining the part, I discovered it was optically not use able—I had not thought out the optical design properly before machining it. Amazingly enough, however, it was exactly the right size for use with this water immersion lens on the BHA! The BHA has a better overall illumination system than the CH, and the resulting images this way are outstanding.
Unfortunately the LOMO Biolam or Mikmed microscopes that are equipped for phase contrast do not have a phase ring that matches this LOMO objective. I machined a phase ring for it similar to the one described above for the Olympus BHA, and it works very well.
The LOMO objective has excellent resolution near the centre of the field with noticeable curvature of field. This objective is not spring loaded, so great care must be taken to avoid ramming it into preparations!
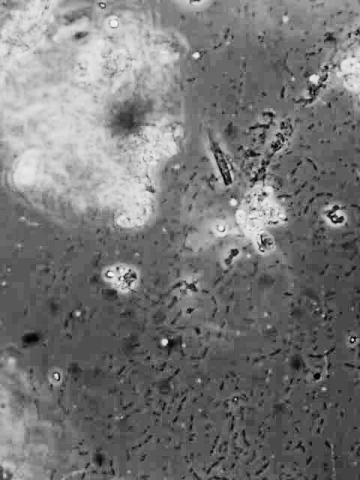
The image to the left of this text was taken using the LOMO microscope with the water immersion phase objective described above. It is difficult to capture an image that really does justice to the visual impression, because of the extremely shallow depth of field at this high power. Only a few bacteria on the image are sharply in focus because of this. Still these few are quite sharp.
Observing mobile bacteria with this lens is truly amazing! If the preparation contain food particles they will move around it in great swarms! It is amazing that a so called "simple" cell is able to move in such an organised fashion.
Non motile bacteria that are not attached to the slide or cover slip are also in motion from the so called "Brownian motion". Bacteria are so small that they are bounced around by statistical variation in the number of collisions they undergo with water molecules!
Although most people like to use phase contrast with white light, phase objectives are normally designed for use with green light. One can do this with tungsten filament illuminators using green filters. With light emitting diode illumination systems it is best to use a green light emitting diode. The LED produces light within a narrow band of wavelengths—ideal for this purpose.
Observing Living Bacteria with Dark field:
Another way to observe living bacteria is with dark field illumination. Dark field condensers project a hollow cone of light onto the subject so that the objective cannot take in the edge of this cone. When using dark field illumination to observe small objects like bacteria it is best to use oil immersion dark field condensers. These typically have an "inner aperture" of about 1.1 or so. This generally permits their use with any high power high numerical aperture dry objective, and it also permits their use with oil immersion objectives that are equipped with a special iris.
Oil immersion dark field condensers are not especially difficult to locate. The bacteria appear as little white dots. Motile ones again move all over the field at a rather high speed, and Brownian motion is also apparent.
Observing Stained Bacteria:
Instead of utilising phase contrast, bacteria can be stained with dyes. This, of course kills them, so one is observing "late" bacteria rather than living ones.
The stained bacteria need to be in a medium that has an index of refraction near that of optical glass and immersion oil—around 1.515.
Because cover slips and immersion oil both have an index around 1.515, it is not necessary even to use cover slips. One can simply place the bacteria on a slide, fix them to the slide, stain them, remove the excess stain, dry them, and then put a drop of immersion oil directly on the slide. Some objectives are especially designed for doing this—their focal distance is so short that they cannot be used with cover slips at all!!
When permanent preparations are required, after the bacteria are stained and the preparation dried, a drop of mounting medium can be applied to the slide, and a cover slip placed on it.
The standard bacterial staining technique that is used in most laboratories is the following:
- 1. Spread a thin film of material containing bacteria over the surface of a slide. If the bacteria be in a concentrated culture they will need to be diluted.
- 2. Dry the thin film in air.
- 3. Pass the slide through a flame a few times with the film side up. This will cause the bacteria to adhere to the slide. (Sometimes this is accomplished by using certain chemical materials such as formalin.)
- 4. Put a drop of solution of a standard dye such as gentian violet, crystal violet, fuchsin, etc. on the slide. Allow from 15 seconds to two minutes for the staining process.
- 5. Wash off the stain with water. It is best to use distilled water, because tap water often contains many dissolved minerals.
- 6. Air dry.
- 7a. Unless the slide is to be kept permanently, place a drop of immersion oil on the slide and observe.
- 7b. To make a permanent preparation place a drop of mounting on the slide, add a cover slip and press down to exclude air.
The dye solutions are typically used at concentrations between 0.1% and 1%.
Microbiologists commonly use something called the "Gram Stain". It is only slightly more complex than the standard staining method described above.
The Gram staining procedure involves first staining the bacteria with a pararosanilin dye such as gentian violet. It is then treated with aqueous iodine which acts as a mordant to fix the dye to the bacteria. Then the bacteria are rinsed with an organic solvent, usually alcohol, to remove unfixed dye. Then the bacteria are subjected to a second stain having a different colour to make the bacteria that lost the first dye visible.
The following procedure is typical:
- 1. Make a thin film of bacteria, dry it, and then pass the slide through a flame as in the previous staining procedure.
- 2. Apply crystal violet or gentian violet to the bacterial film.
- 3. Add two or three drops of 5% sodium bicarbonate solution and wait two minutes. Then rinse with water until no more stain washes out.
- 4. Cover with "Gram's Iodine" for about 1 minute. (see below)
- 5. Wash with either 50-50 acetone-alcohol or 95% alcohol until solution does not colour.
- 6. Apply the second stain, safranin or carbol fuchsin is commonly used for this. Then rinse with distilled water and air dry.
- 7. Either add a drop of immersion oil and examine or else make a permanent preparation as described above.
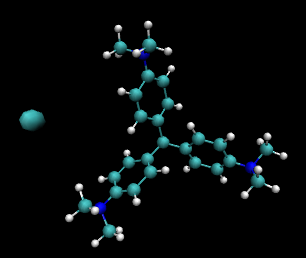 The crystal violet or gentian violet stain is made by dissolving the solid in alcohol. The image to the right of this text is a representation of its molecular structure drawn using the molecular display program from the University of Illinois called vmd. The large cyan ball on the left of the image represents the chloride ion. The small cyan balls represent C, the white ones H, and the blue ones N. (This material binds strongly to many things because the structure is really a strongly stabilised carbocation.)
The crystal violet or gentian violet stain is made by dissolving the solid in alcohol. The image to the right of this text is a representation of its molecular structure drawn using the molecular display program from the University of Illinois called vmd. The large cyan ball on the left of the image represents the chloride ion. The small cyan balls represent C, the white ones H, and the blue ones N. (This material binds strongly to many things because the structure is really a strongly stabilised carbocation.)
Gram's iodine is made by dissolving 0.5 grams of iodine in a mixture of 1 gram of potassium iodine and 5 mL of water. When the iodine is completely dissolved add water to make 150 mL. It is best to add a pinch of sodium bicarbonate or carbonate to be sure that this solution is not acidic.
The gentian violet or crystal violet will stain most bacteria. The iodine is a mordant. It fixes the stain in some bacteria so that it will not be removed by the alcohol or alcohol acetone mixture. The second stain is of a different colour to be able to locate the bacteria that lost the stain in the alcohol or alcohol acetone rinse step.
The bacteria that retain the original dye are called Gram positive. These bacteria have a fairly thick wall of a polymer of sugars and amino acids called peptidoglycan. Gram negative bacteria have a much thinner layer of this type of material, and they have an outer layer of lipids. When these bacteria are treated with alcohol or alcohol-acetone mixture the lipids are stripped away, taking most of the colour with them. Microbiologists commonly divide bacteria into two groups, Gram negative and Gram positive on the basis of this stain.
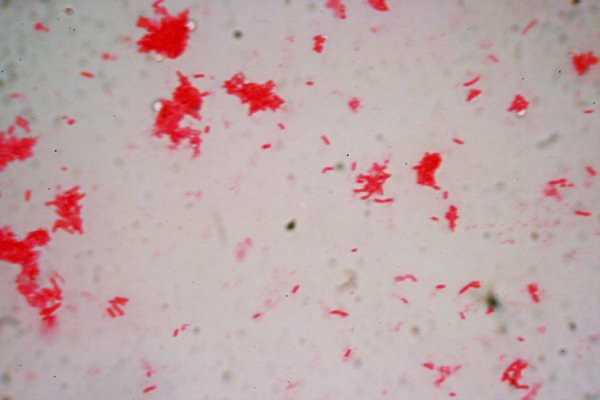
The image below this text is a culture of unknown bacilli. The sample was made in a kitchen instead of laboratory to demonstrate that preparations like this can be made without special equipment, except, of course a good microscope. The image was obtained using a Canon digital single lens reflex and an Olympus 100X 1.3na flat field apochromat.
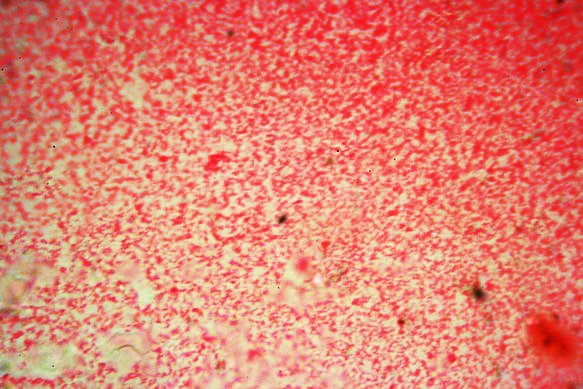
The next image, the one below this text, is also produced in a kitchen! It is of unknown cocci using the same microscope and camera system as used on the previous image. Because they are very tiny they look just little dots!
For observation of any type of stained specimens it is best to use a very high quality oil immersion lens with as high a numerical aperture as possible. It is generally best NOT to use a oil immersion phase objective for this purpose, even with an ordinary condenser. The instrument should also be equipped with an oil immersion condenser.
High power high numerical dry objectives can work quite well too, especially for larger bacteria. Unfortunately, however, these objectives are typically at least expensive as oil immersion ones.
Because observing bacteria involves observing objects at the limit of optical resolution one needs to do everything possible to achieve the highest possible resolution.
All comments to the authors
via
Robert
Pavlis
are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
©
Microscopy UK or their contributors.
Published
in the July 2008 edition of Micscape Magazine.
Please
report any Web problems or offer general comments to the
Micscape
Editor
.
Micscape
is the on-line monthly magazine of the Microscopy UK website
at
Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All
rights reserved. Main site is at
www.microscopy-uk.org.uk
with full mirror at
www.microscopy-uk.net
.
 The crystal violet or gentian violet stain is made by dissolving the solid in alcohol. The image to the right of this text is a representation of its molecular structure drawn using the molecular display program from the University of Illinois called vmd. The large cyan ball on the left of the image represents the chloride ion. The small cyan balls represent C, the white ones H, and the blue ones N. (This material binds strongly to many things because the structure is really a strongly stabilised carbocation.)
The crystal violet or gentian violet stain is made by dissolving the solid in alcohol. The image to the right of this text is a representation of its molecular structure drawn using the molecular display program from the University of Illinois called vmd. The large cyan ball on the left of the image represents the chloride ion. The small cyan balls represent C, the white ones H, and the blue ones N. (This material binds strongly to many things because the structure is really a strongly stabilised carbocation.)


