
IS IT AN ANIMAL?
IS IT A PLANT?
IS IT A MYXOMYCETE?
Manuel del Cerro and Dietmar R. Krause
Princeton, NJ, USA
FINDING. During the month of May of this year one of us (DRK) noticed small, intensely red spots on a broken tree branch at the Rocky Top Dog Park, South Brunswick, NJ (GPS 40.390673,-74.592737). Later we noticed patches of similar red dots on the surface of fallen tree trunks (figs 1 & 2).

Figure 1. Areas covered with red dots can be seen on parts of the fallen tree trunks (asterisks).

Figure 2. Closer view of the area shown on the right side of figure 1 showing the clusters of red dots.
MACRO EXAM. Macro pictures showed the dots to be about 1 mm in diameter (figs. 3 & 4). The question was: were the dots eggs of snails or insects, or were they some type of a fungus (including slime molds, a.k.a. myxomycetes. More about this later)?

Figure 3. Macro photo of the red dots.

Figure 4. A similar area showing the size of the dots.
For microscopic examination some of those specks were removed from the underlying bark and placed over glass slides with drops of spring water on them. To spread the contents of the sample the preparation was covered and the cover glass gently taped with the closed end of a rubber bulb of an eyedropper. Next, a drop of lactophenol-cotton blue was placed at the margin of the cover glass and the stain allowed to slowly diffuse under the cover (Leck, 1999). The lactophenol cotton blue mixture serves two functions; the phenol and the lactic acid fix the cells, killing them and preserving their structure at the same time. The cotton blue is very effective as a stain that makes visible many cell structures.
A video showing a slightly different way of using lactophenol cotton blue is available in YouTube (Microrao, 2011).
PHOTOMICROGRAPHY. Photomicrography was performed by using a 3.3x Olympus projection ocular that presented the image to a Nikon D80 DSLR camera body operating on manual mode. The captured images were downloaded into a 17 inch MacBook Pro lap top computer, and displayed using Apple’s iPhoto program. To measure the features of the preparation we determined magnification by photographing a stage micrometer, bringing its image to the computer screen and measuring it with a metric ruler (figure 5).

Figure 5. The 17 inch screen of the MacBook Pro displays the picture of the stage micrometer. The field width is 550 micrometers. The magnification is the same used to photograph the specimen showed in figure 6 (10x objective, 3.3x ocular).
OBSERVATIONS. The addition of a drop of stain to the border of the cover glass allows a slow diffusion of the stain and makes the staining process very gradual so that it was possible to see stained and unstained portions of the sample in the same microscopic field (figure 6).
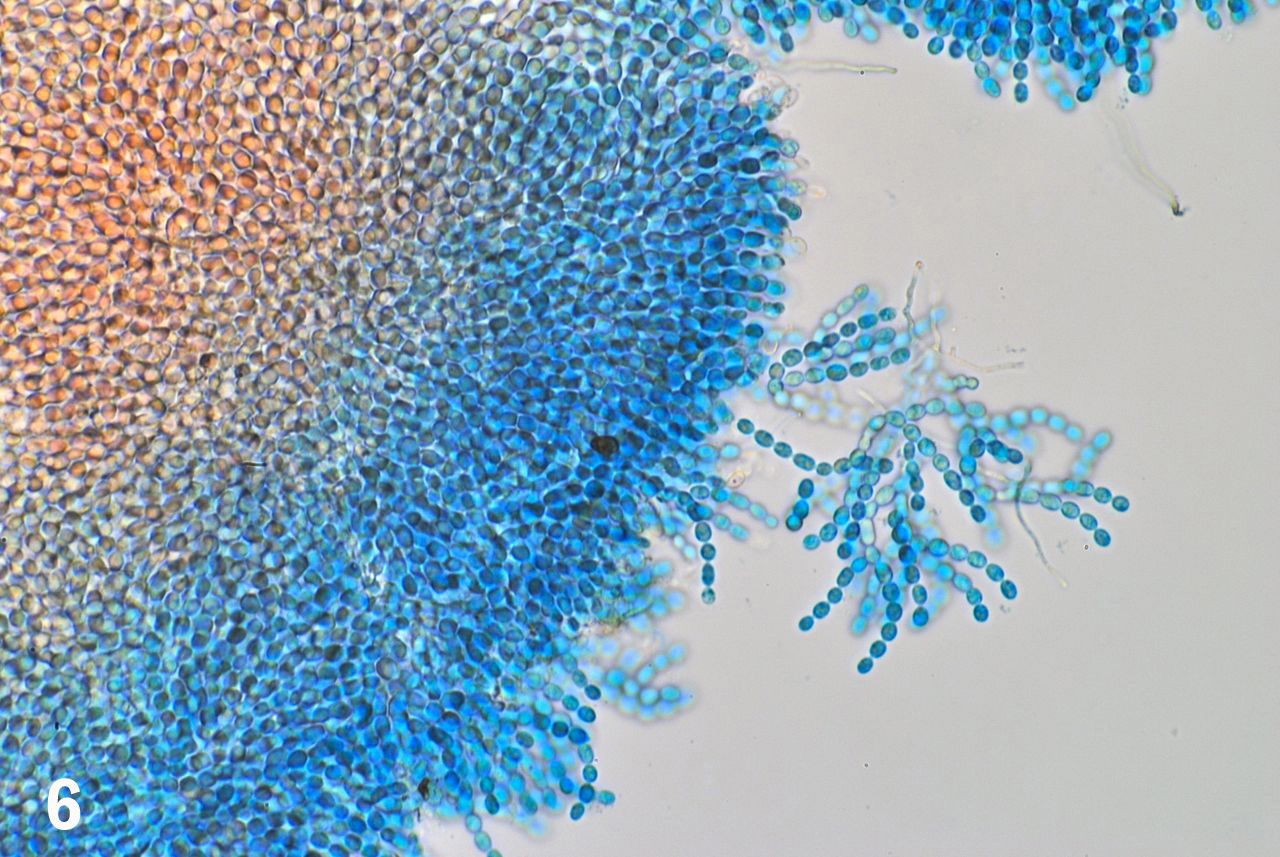
Figure
6. The stained portion of the preparation stands out in light blue
while the tissue still unstained retains its original coloration.
Magnification and field width are the same as in figure 5.
Even at this low magnification we had the impression that we were dealing with “plant” cells, as opposed to the samples being the eggs of some unidentified invertebrate. The sample was composed of an innumerable collection of densely packed, globular cells rather uniform in size and shape. Strings of these cells, arranged as pearls in a collar were visible at the free margins of the sample; some of the strings were detached from the central mass by the pressure applied to the cover glass when making the squash preparation (figure 6).
To better demonstrate the end-to-end connections of the cells forming the strings we used a 100x Olympus SPlan oil immersion objective (NA 1.25, TL 160 mm/0.17). The magnification changer of an Olympus BHS was set at 1.5x, effectively transforming the objective into a 150x lens. We were aware that this set up was to enlarge the image into the realm of empty magnification, but preliminary testing showed that it served the purpose of making the end-to-end connections between cells self evident (figure 7) and that it showed to advantage the occasional side-connections that occur at the point where the cell strings bifurcate (figure 8). We took advantage of these greatly magnified images to measure the cells on the computer screen by placing a metric ruler on individual cells as seen on the screen and converting the reading to micrometers. The measured cells averaged 6.8 micrometers in length along their long axes.
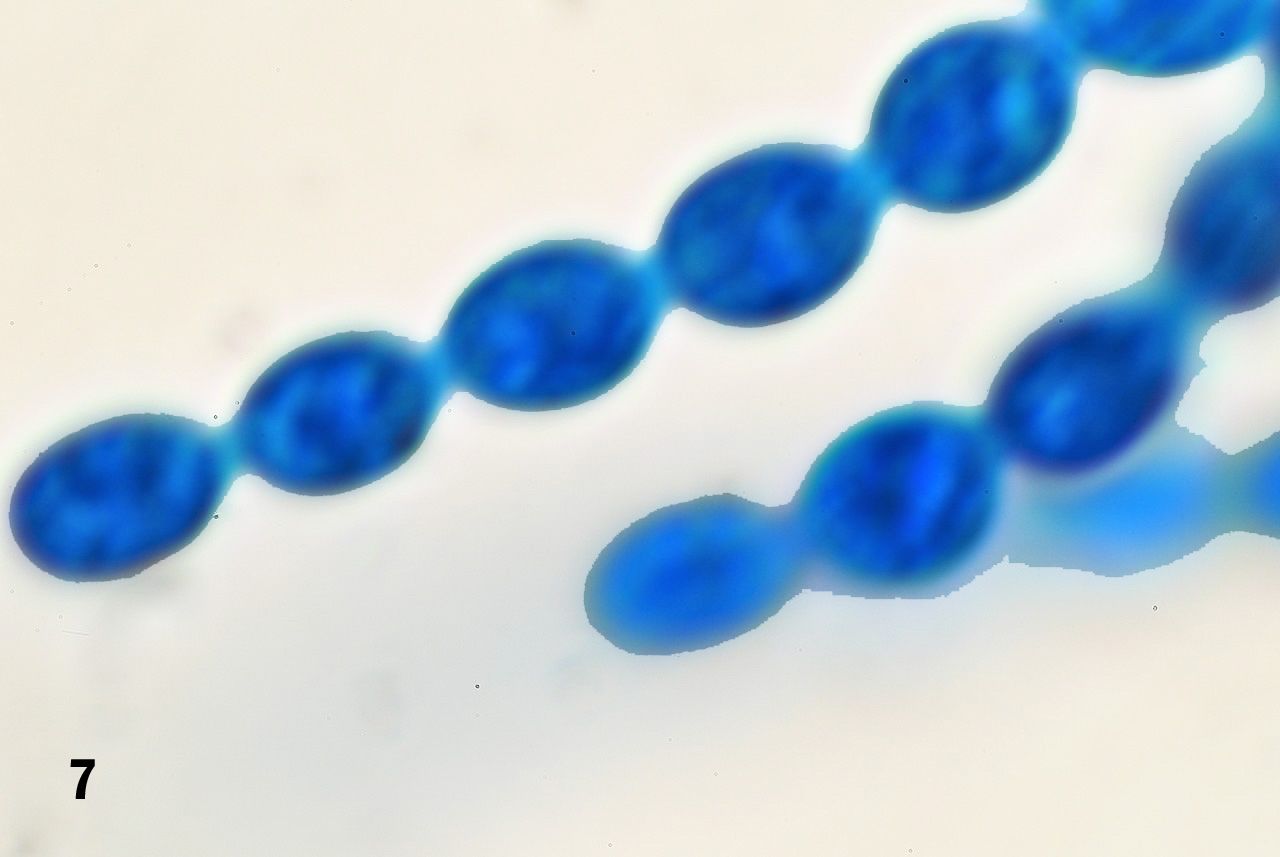
Figure 7. Strings of cells attached in an end-to-end fashion.
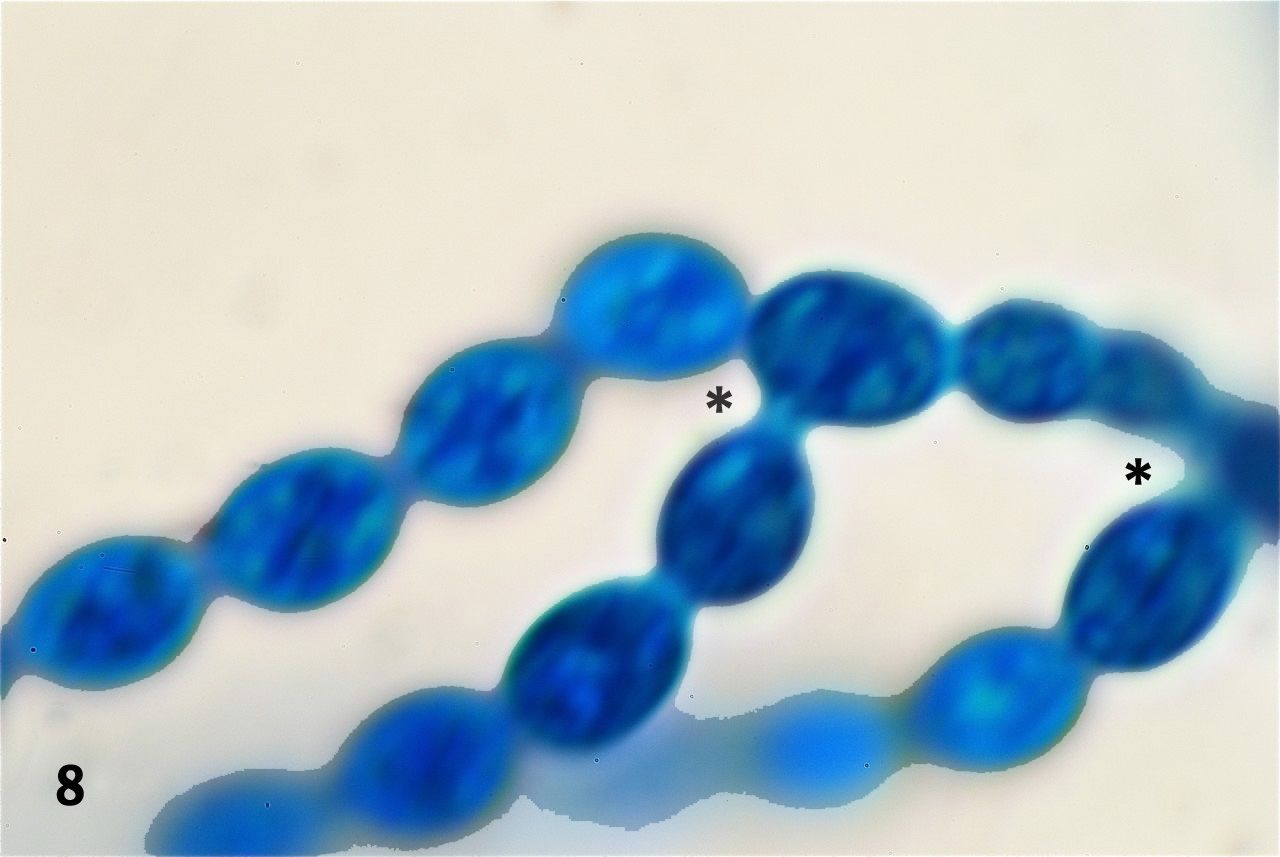
Figure 8. Occasional bifurcations occur along the strings of cells. At these points one cell develops besides its two end-to-end attachments, a side attachment to a third cell. Two of those instances are seen in this picture (asterisks).
The squashed preparations showed two cell types. Besides the overwhelming majority of lemon-shaped cells just described, there were others quite different in shape and arrangement inside the tissue mass. These were, thin, elongated cells projecting towards the outside of the tissue mass (figure 9). These are elements of the capillitium, the system of threadlike elements found within the spore mass of the fruiting bodies of many myxomycetes (Stephenson & Stempen, 1994).
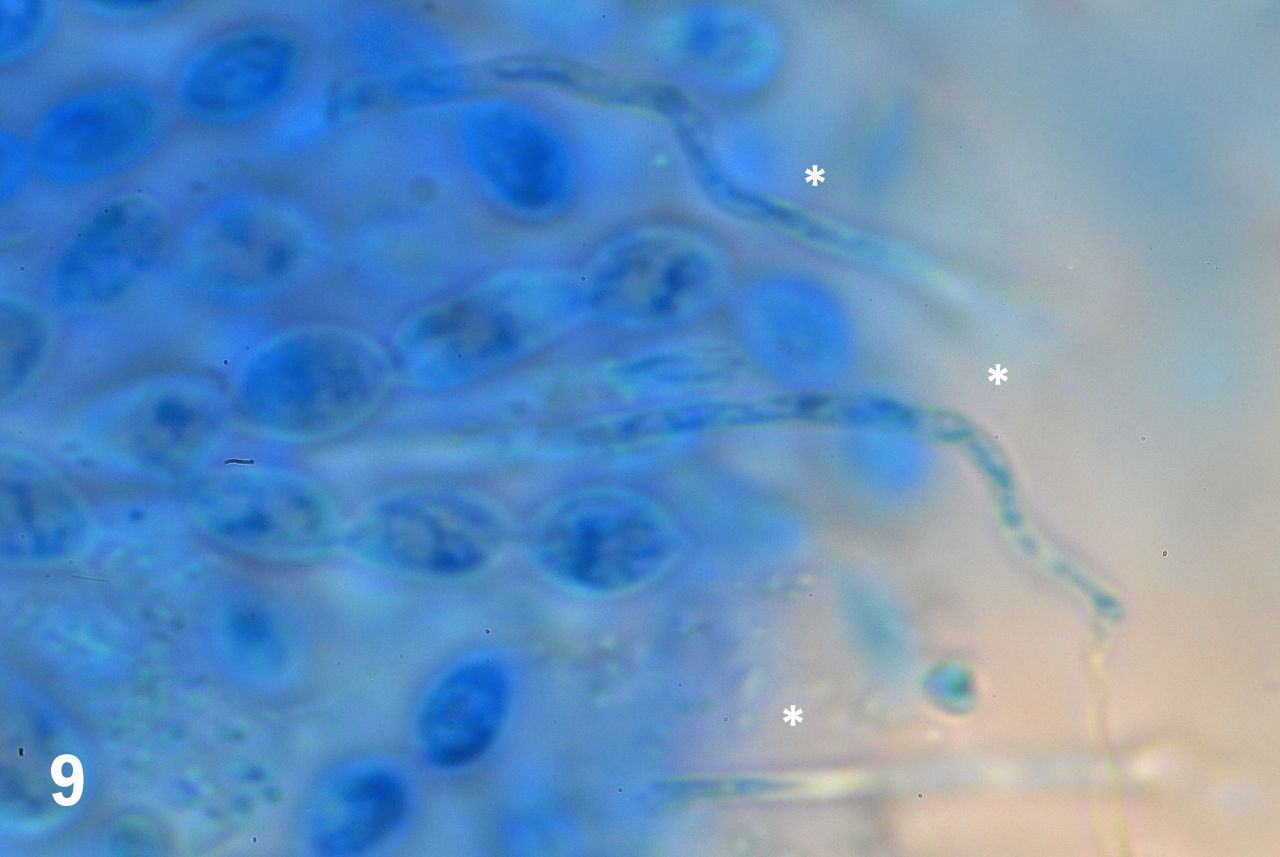
Figure 9. Elongated elements radiate from the cell mass (asterisks).
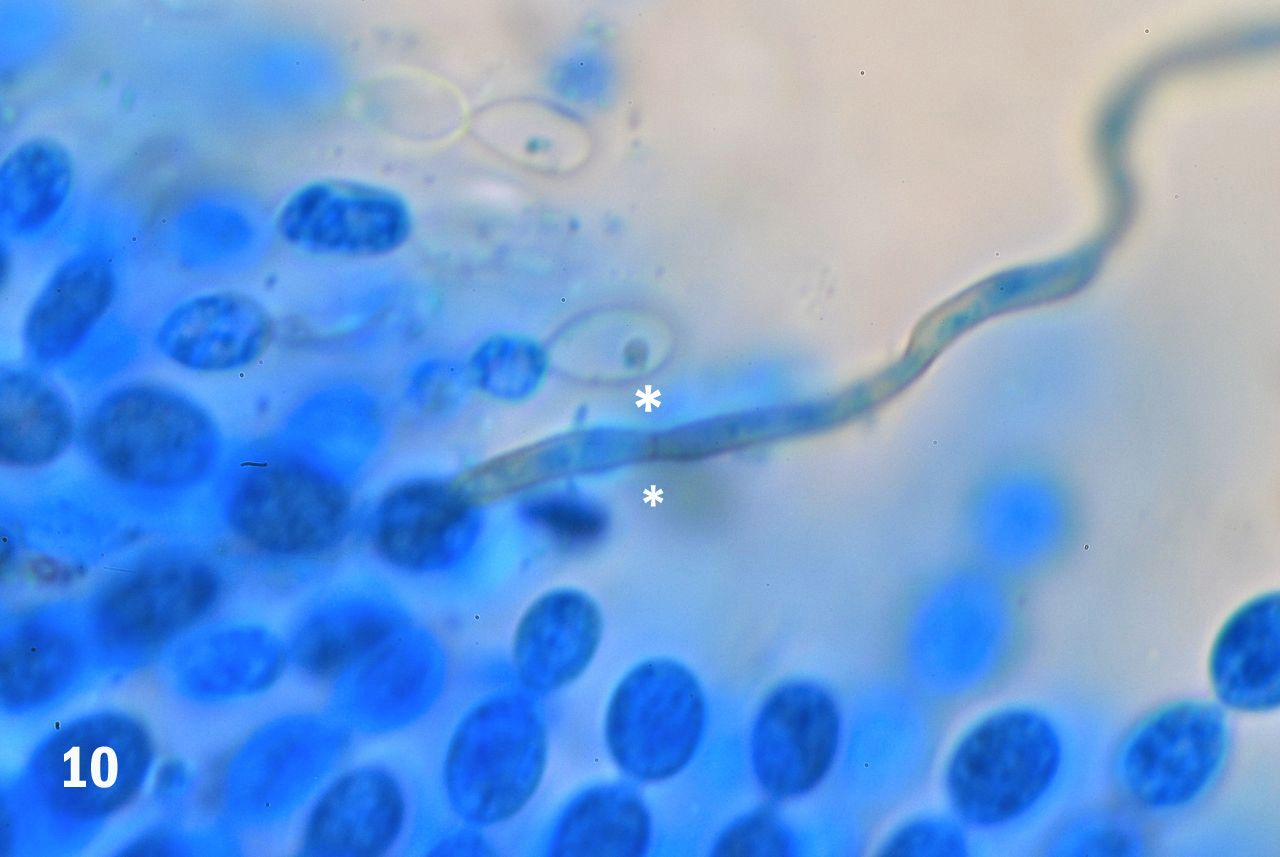
Figure 10. A single strand of the capillitium showing a septum separating two contiguous elements (asterisks).
COMMENTARY. On the basis of these observations we feel reasonable to assume that the red dots growing on the surface of the logs are the fruiting bodies of a cellular slime mold. Slime mold? “Ay, there's the rub” as Mr. Hamlet used to say! For taxonomically speaking, what exactly is a slime mold today, at the start of the second decade of the 21st Century?
During many decades of the 19th and the 20th centuries zoologists and botanists battled over the appropriate location of slime molds within the “Tree of Life.” The zoologists argued that the slime molds are animals since they start their living cycle as free ranging, single-cell amoebas that feed on whatever bacteria they find on their way. Botanists countered that these amoeba converge by the thousands to form a non-motile, spore-bearing being with more than passing resemblance to some molds; therefore they should be considered plants. The struggle ended, apparently by exhaustion, and the slime molds were left to occupy a place among the fungi.
Unfortunately, peace was not lasting. The cataclysmic changes that took place in the taxonomy of all living things in the 1980s and beyond, did not leave the slime molds untouched. First, the entire class of the fungi was taken away from the kingdom of plants and given a kingdom of its own: The Kingdom Fungi (see Armstrong 2011 for an overview). The slime molds went to the new kingdom, together with the rest of the fungal universe. That didn’t last either. Newer studies suggested that there are some fundamental differences between the slime molds and the “true fungi” and that these differences were important enough to warrant expulsion of the slime molds from the Fungi Kingdom. And on they went, the slime molds. Where to? To the Kingdom Protista (Anonymous, 2007), under the name of Eumycetozoa. That is as far as we will venture into this intimidating, ever changing world of scientific nomenclature. One thing is sure: the slime molds, are not molds! They are not animals, and they are not fungus. Incidentally, they are not always “slimy” either. Some authors even consider them “elegant” (Kaneko-Binkley, 2003)
Oh, well!
We wanted to see if our presumed slime molds resembled some of those seen in the literature at our disposal. They did; they do have a resemblance to the so-called Insect-egg Mass Slime, Leocarpus fragilis (Lincoff, 1997). Of course, we are not claiming that this is L. fragilis, only suggesting that it bears strong resemblance to it and it could therefore be it or a related species. One thing is undeniable, however, even a casual stroll at a dog park can take amateur microscopists to totally unexpected realms.

FEEDBACK
Comments to the authors are welcomed (via Manuel del Cerro) will be gratefully received.
SOURCES
• Anonymous (2007) Eumycetozoa.
• Armstrong, Wayne P. (2010) Slime Molds. Wayne's Word.
• Armstrong, Wayne P. (2011) The Five Kingdoms of Life.
• Kaneko-Binkley, Susan (2003) The Elegance of Slime Molds. Minnesota Conservation Volunteer.
• Leck, Astrid (1999) Preparation of Lactophenol Cotton Blue Slide Mounts. Community Eye Health, 12(30):24.
• Lincoff, Gary H. (1997) National Audubon Society Field Guide to North American Mushrooms. Alfred A Knopf, New York, pp 846-847).
• Microrao (2011) Lactophenol Cotton Blue Mount.
• Stephenson, Steven L. and Henry Stempen (1994) Myxomycetes. A Handbook of Slime Molds. Timber Press, Portland, OR, p. 28.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the July 2011 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk .