|
Some Ramblings on Micro-Technique, Part 1: Spring Ponds and Ditches by Richard L. Howey, Wyoming, USA |
After long winters, I look forward to early spring and late autumn. Not summer, you might ask? Definitely not! High temperatures of 65 F. to 70 F. are just about ideal at this altitude of 7,200. A temperature of 75 F. , I can tolerate, but anything much above that I regard as a raging inferno. Furthermore, in the summer, there ascends from the foulest, vilest bowels of Hell those creatures that only Satan could have “intelligently” designed–the mosquitoes!! And, as the temperatures rise, the despicable species that hatch and breed are increasingly those that can carry the West Nile virus and this is a malady which can ruin your entire summer, if it isn’t fatal first. These wretched creatures have a long history of being detested.
Against Mosquitoes
Squealshrilling
Mosquitoes, fraternity lost to shame,
Obscene
vampires, chittering rider of the night:
Let her
sleep, I beg you! and come
(If you must
come) feed on this flesh of mine.
Oh useless
prayer! Must not her body charm
The wildest,
most heartless, most insensate beasts?)
Yet hear me
devils, I have warned you:
No more of
your daring,
Or, you
shall smart from the strength of my jealous hands!
–Meleagros
translated by Dudley Fitts, Poems from the Greek Anthology, New Directions, New York 1956, page 25.
So, from mid-June to mid-September is a good time to retreat to my basement laboratory space and there work either on wonderful preserved mysteries such as echinoderms and tunicates or investigate the pond samples collected before mid-June and anticipate collecting more in mid-September. This year we had more snowfall and rain than we’ve had during the previous 5 or 6 years, so early May, I was out swinging my long-handled net through ditches alongside the road. In fact, there was flooding along the river from the melt off of the snowpack which this year was 233 percent of normal. I found the usual suspects–cyclops, rotifers, nematodes, mayfly nymphs, mosquito larvae, small water beetles, bright red water mites daphnia, ostracods, cladocerans and even a very young dragonfly nymph. This year I had decided that I was going to concentrate on algae and protozoa and, after letting the samples “ripen” for a few days, I was not disappointed. In the river sample alone, I found numerous small diatoms, a few desmids (primarily Closterium), aquatic oligochaetes, Spirostomum, Bursaria truncatella which can swallow a Paramecium whole, the elegant rotifer Asplanchna, and that splendidly alien creature, Volvox.
There appeared, of course, the ubiquitous Paramecia of which I immediately started some cultures, since they are always worth studying and because they can be maintained long-term in large quantities and so make excellent subjects for testing various medications which quack physicians have prescribed for me and which I stopped taking as a consequence of undesirable side-effects. My view is waste-not, want-not–try them out on Paramecia.
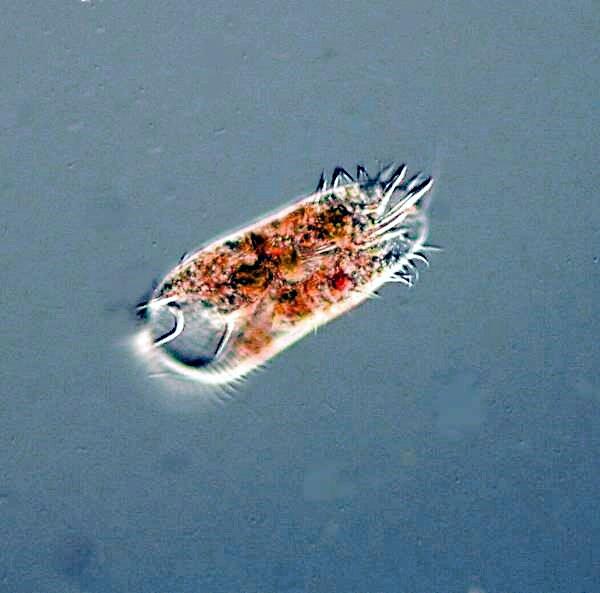
There were also the usual hypotrichs, Oxytricha, Stylonychia, and Euplotes, but also a very large species which I had not seen before.

Hypotrichs are cirriates, that means they possess bundles of fused cilia called cirri rather than just “simple” cilia. Hypotrichs are protozoa which have overdosed on caffeine. If you’re going to study these organisms over a long period, it might be desirable to get yourself a prescription for tranquillizers. Whether you take them yourself or feed them to the hypotrichs is up to you. They dart and jerk, and twist and turn, and then crawl and creep along and you never know quite what they are going to do.
However, for real hyper-motility try watching Urocentrum turbo; this is a ciliate on “speed”. Imagine an upside-down pear with a corset of cilia around its mid-section and a the stem which is a bundle or tuft of long cilia. At the tip, they secrete a tiny amount of adhesive and then like a tiny turbine or dynamo, they spin like mad, drugged dervishes and then just as you turn your lens turret to increase the magnification, they whirl away out of the field of view, faster than the speed of sight. Here we encounter one of the great frustrations of the microscopist; we have an intriguing organism which presents us with a series of puzzles and challenges and, at the same time defies our attempts to observe it in detail as a living functioning entity. Perhaps, if we had a high-speed microscope video-camera, we might be able to capture some information which when viewed in slow motion would give us some new insights. However, trying to track such erratic organisms would be a technological nightmare and there is also the factor that such equipment is expensive.
Our attempts to investigate and understand various organisms are inevitably a matter of piecing together large amounts of data acquired by means of every sort of methodological and technological trick we can think of and even then, organisms such as Paramecium and Tetrahymena which have been extensively studied can still confront us with surprises or pose questions to which we don’t yet have answers. For example, it is still a matter of debate whether or not Paramecia form cysts.
Trying to study the morphology of small, rapidly moving organisms like Urocentrum inevitably involves compromises. Mechanical means such as using a network of cotton fibers or increasing the viscosity of a drop by means of methyl cellulose are virtually useless. Narcotizing ciliates is, at best, a hit-and-miss procedure since what seems to work well for one organism will be ineffective or highly distorting for others. Furthermore, the alteration of behavior is inescapable. In addition, most of the substances that are most likely to be effective are either highly toxic and/or unobtainable by the amateur because they are restricted substances.
So, with an organism such as this, what we are left with is trying to find a quick acting killing and fixing agent that produces a minimum of distortion. Again, many fixatives are extremely toxic, dangerous, and difficult to acquire. Osmium tetroxide (deadly!), mercury compounds (deadly!), uranyl acetate (deadly!), picric acid (highly explosive!), cyanide compounds (deadly!), strychnine (deadly!), arsenic compounds (deadly!) have all been used by microscopists–may they rest in peace–but fortunately, these are almost completely unobtainable by the amateur. These compounds are simply too dangerous to work with unless one has proper training and proper laboratory facilities.
So, what are we amateurs left with? One of my favorites is booze. Go buy a nice bottle of single malt Lagavulin (for about $95 these days) and while you’re at the liquor store, pick up a bottle of their cheapest rotgut vodka and a bottle of seltzer. The Scotch is for you to sip on while you’re waiting for the vodka to take effect on the Urocentrum. Use about a 10% solution to begin with and then experiment from there. If this highly sophisticated procedure doesn’t give you wonderfully tipsy, observable specimens of Urocentrum, the Lagavulin will soften the blow and make you quite ready to reinitiate the search for a proper anesthetic the next day. (If, however, you are a complete Philistine, you can give the Lagavulin to the Urocentrum and save the vodka for yourself. If this turns out to be the case, don’t ever expect to present a paper before the Edinburgh Microscopical Society!)
As for the seltzer, it is sometimes effective when applied to rotifers and other small aquatic invertebrates.
Now, if the organisms you’re trying to anesthetize don’t cooperate, we have several other options, one of which may very well be in your medicine cabinet–magnesium sulfate (a.k.a. Epsom salts) and the commercial brand you can buy in a drugstore or discount store is, for our purposes, as good as or better than the “pure” stuff. Every aquatic biologist should keep some of this on hand; it’s quite wonderful (if you’re patient and methodical) for relaxing a fairly wide variety of both marine and freshwater invertebrates so that they can be killed and preserved extended. (We’re a ruthless, bloodthirsty lot, we biologists.) Magnesium sulfate used on protozoa is, as you can well imagine, a trial and error issue, but sometimes it does work.
The real point is that when you are out collecting in good locations, you are assured a wide variety of fascinating organisms and so you need as extensive an arsenal as you can put together to meet the challenges in your lab of studying the organisms which most capture your fancy at any give time. So, what else can we add that’s readily available? Most amateur biologists have managed to acquire some formalin or know someone who can provide them with a small quantity of the nasty stuff and admittedly , it is not only unpleasant, but a health hazard–so handle it carefully, shield your eyes and skin, and learn how to hold your breath for at least 6 hours (practice yoga). Go to your local hardware store and buy a small can of their strongest wood alcohol (methyl alcohol). Mix 10ml. of full strength formaldehyde with 10 ml. of full strength alcohol (again, exercise caution–it is highly poisonous and highly flammable!). Make up a solution that’s 50/50. Use the solution at full strength. This is best done by placing a few milliliters of a rich solution of protozoa into a small vial and then (using a disposable pipet), squirt approximately an equal amount of the fixative into the vial. This works, of course, only for certain ciliates and flagellates. The formula was passed along to me by a late colleague who was a first rate protozoologist and cell biologist who admitted that he didn’t know why it worked, but seemed satisfied that it did.
Simple, effective techniques are always satisfying unless you are one of the compulsive sorts that relishes going through a 30 stage procedure that requires 5 days and dozens of reagents. A pre-eminently simple technique which is effective in killing some species of rotifers in an extended state involves placing a sample abundant with rotifers in a small heat-resistant dish or beaker and then suddenly pouring boiling water over them. Once the rotifers have settled to the bottom, pour or pipet as much of the water off as possible with minimal disturbance. Now you need to add an appropriate hardening and fixing agent, such as a dilute solution of formol or a mixture of formol and alcohol and some researchers like to add a bit of acetic acid as well.
If you have an arrangement with a scientific supply house that will sell you small amounts of relatively “safe” materials or an acquaintance who has access to chemicals, then your possibilities expand significantly. [Perhaps a Micscape consortium could be established with a dealer in the U.K. and one in the U.S. to start with, who would agree to supply small quantities of chemicals on an approved negotiated list to members who were willing to pay a modest annual fee. Special requests could be made and, if approved, could be added to the list. This strikes me as feasible because the amounts of a chemical that microscopists require are usually very small and secondly many amateurs would be willing to pay a bit of a premium to obtain such materials for legitimate and exciting research rather than being placed in the position of being denied access to such reagents altogether.]
For starters, let me mention a couple of classics that microscopists have used for at least a century for anesthetizing protozoa. The first is Nickel sulfate which is somewhat erratic, but with persistence can be very useful. Much the same can be said for Potassium iodide. When I get frustrated at not getting the results I want, I have to remind myself of just how wonderfully complex the biological systems of, for example, a Paramecium are. Naturally we get frustrated, but when we find a technique that works, even some of the time, we should exult.
Copper salts are also worth investigating. If one is resourceful, one can often find local commercial sources for Copper sulfate which has been extensively used for controlling “blooms” of various sorts of undesirable aquatic organisms. Some investigators have reported good results with this reagent applied to aquatic invertebrates. Personally, I prefer Copper chloride and especially Copper acetate. Copper acetate, depending on the concentrations used, can be employed as an anaesthetic or as a very delicate killing agent which must then be followed up with an appropriate fixative.
In the early spring, I often find in the cold water of Lake Sodergren, about 20 miles southwest of Laramie, several species of tintinnids which aren’t present in summer collections when the water is a bit warmer. I suspect that they migrate to deeper, colder parts of the lake. They secrete a lorica or “house” which is often adorned with tiny bits of sand and/or other debris. They extend their anterior portion out of the lorica when feeding, but quickly contract when disturbed. There is a wide variety of ciliates that are highly contractile and these pose special challenges to anyone trying to study them in detail. Several are quite common, widely distributed, and relatively easy to maintain in cultures or samples, but getting a close look at them demands ingenuity.
One of the easiest to culture are the species of the genus Spirostomum–the “micro-whales”. The large species, namely, S. ambiguum and S. minus are particularly impressive and are certainly the giants among the ciliates. Most of the great whales are plankton feeders, eating krill in enormous numbers; similarly Spirostomum devours huge numbers of bacteria and tiny flagellates. These remarkable creatures can reach a length of over 3000 microns and contract to 1/3 or 1/4 their size so rapidly that one can only see the Before and After state like those in advertisements of human diet pills. It has been stated the this is the most rapid contraction in the biological world as we know it at present. Some audacious biological supply houses sell slides of Spirostomum–preserved, stained, and semi-extended. Don’t get me wrong, such slides can be useful, but they are never a substitute for observing the living organisms.
Virtually every substance I have tried on Spirostomum has resulted in extreme, violent contraction and massive distortion. One saving grace is that the large species generally move slowly enough that one can observe them fairy closely especially as the water evaporates and the cover glass begins to exert a bit of pressure. However, as the water continues to evaporate, the specimens will begin to undergo a series of twitching contractions and then lyse (break open). Even this can be instructive, for as the contents flow out through the ruptured membrane, it is often possible to get a good look at the macronucleus which in the case of the two largest species mentioned above is a long beaded chain. S. teres, a much smaller species, has a single ovoid macronucleus.
I have gotten the best results on contractile ciliates and a variety of other protozoa by not even trying to anesthetize them, but by using Harris hematoxylin which kills, fixes and stains all in one operation. It must be used with caution since it contains mercuric oxide which is extremely poisonous, attacks metal instruments, and contaminates containers in which it is used. There is now a version which substitutes Potassium iodate for the mercuric oxide and is said to work as well or better than the version with mercury salts.
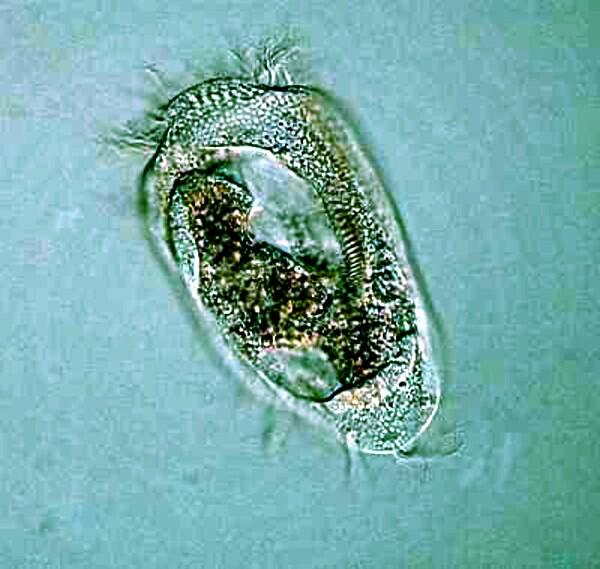
Stentor and Vorticella are somewhat less difficult than Spirostomum and it can be interesting and rewarding to try out some of the anesthetic techniques on them. The marvelous blue-green Stentor coeruleous which is, when extended, trumpet-shaped and also has a long, beaded chain macronucleus.
Vorticella has a large distinctive “C”-shaped macronucleus and a fibril in the stalk called a myoneme or spasmoneme and when the stalk contracts, one can observe how it becomes a tight coil.
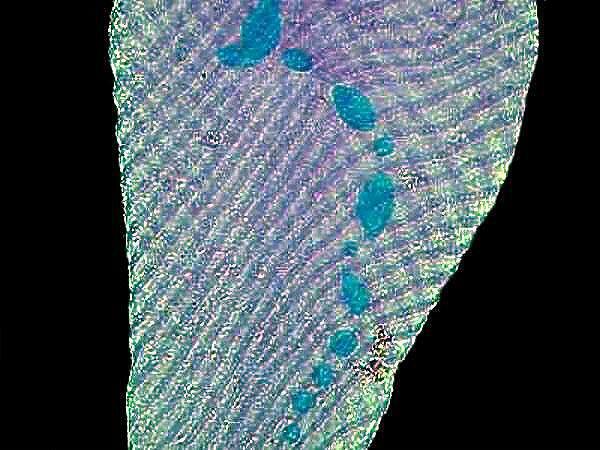
Often the identification of protozoa is aided greatly if one can determine the size, shape, number of macronuclei and the presence of micronuclei, if any. A wonderful reagent for facilitating this is Methyl Green Acetic. By far the best course is to buy this already prepared from a biological supply house; the next best is look up your old chemistry teacher and tell you him know about all of his nefarious, illegal activities, but you’ll keep quiet for 30 cc. of Methyl Green Acetic. As Mark Twain once quipped: “I once sent a dozen of my friends a telegram saying ‘flee at once–all is discovered.’ They all left town immediately.”
In using stains, such as the Harris Hematoxylin or Methyl Green Acetic, I usually administer them on a slide by putting a drop at the left edge of the cover glass and letting capillary action gradually distribute it. This can be of advantage in a couple of ways. 1) A few specimens along the edge will be killed almost immediately and may as a consequence suffer less distortion; however, some species will simply disintegrate, so it’s a matter of trial and error which means that you need to keep good records. 2) As the fixative/stain is slowly pulled toward the right edge, there is a very slight dilution which produces a subtle concentration gradient and some specimens will be frantically swimming and darting just in front of the ‘wave” and will accidentally swim into it, while others will endure to the extreme right edge until the fixative finally overtakes them. The specimens from the middle of the cover glass to the right edge provide the best results with relatively little distortion and distinctive staining, but not overstaining.
Another valuable stain for studying protozoa is Neutral Red, because it can be used either as a vital stain or as a contrast stain depending upon the concentration used. When used as a contrast stain, it reveals in Paramecia what are called Neutral Red granules which are virtually unobservable by other methods. When such a unique and distinctive correlation occurs , one should always be a bit cautious and carefully consider the possibility that these structures may be artifacts resulting from a chemical reaction and are not really genuine cytological features. However, in the case of these granules, evidence strongly supports that they are indeed genuine.
Several years ago, when I was studying some Thecamoebae from a bucket of water and algae sitting under a hanging plant on our patio, I observed that they were voraciously devouring long filaments of algae. I decided to use a very dilute solution of the Neutral Red to stain some of the filaments so I could observe them after and during their ingestion by the Thecamoebae. Here are a couple of images of the results.
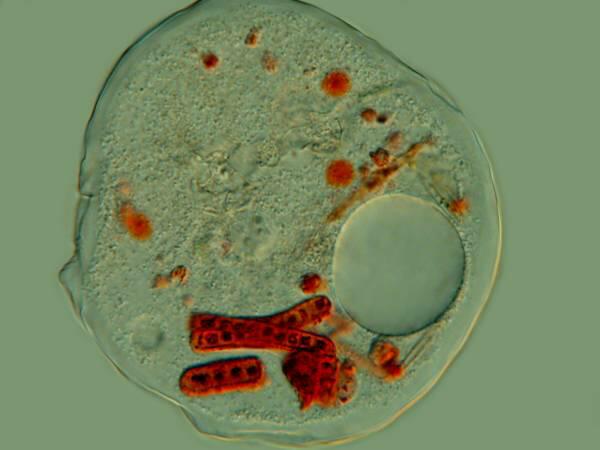
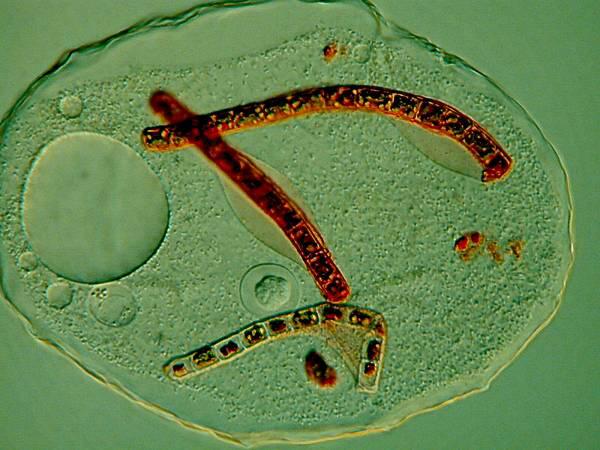
The Thecamoebae don’t themselves seem to absorb much, if any of the stain, so with patience and perserverance, it should be possible to study the full cycle of the digestion of the algae by adding just enough Neutral Red to the culture dishes to stain the filamentous algae without making them unpalatable or toxic to the Thecamoebae.
Micro-technique of this sort is a joy, because it is an opportunity to explore, learn and play. Get a small quantity of powdered carmine; try some of your friends who are artists or into crafts; it’s often amazing the wonderful resources they can provide. Take a tiny amount of the carmine on the end of a toothpick and sprinkle it carefully into a small culture dish of Paramecia or some other sizeable ciliate. For your first attempt Paramecia are probably the best choice because you are virtually certain to get good results whereas some other ciliates will reject the particles. “What are the results?”, you might well ask at this point. Something quite wonderful happens. As the Paramecia are feeding, the carmine particles are swept into the gullet and down to the bottom where a food vacuole is forming. When there is sufficient weight of material in the vacuole, it rounds off and this miniature sphere begins a journey following a highly elongate path within the Paramecium while digesting the material it can utilize. It finally arrives at a point in the pellicle (membrane) where there is a cytopyge, which is essentially an anal pore and there it expels any remaining waste back out into the water. If you have used a sufficient amount of carmine, the food vacuoles will be a brilliant red and if you are very patient you can follow their course through the organism.
If you can’t obtain powdered carmine, you can try India Ink which is basically carbon particles suspended in water which is why this type of ink frequently clogs fine-pointed fountain pens–some of you out there must remember fountain pens! So, if you’re lucky, in some cases, these tiny insoluble particles may produce some black food vacuoles for you. However, an even more amazing application is using India Ink with certain kinds of micro-algae. A significant number of such algae have the surprising trick of encasing themselves in a gelatinous membrane which is virtually invisible. The simple counter-trick of using the ink to provide a gray-black background of tiny particles which, in part because of Brownian motion, are bumping up against this membrane, show it off in a spectacular manner. If you have high contrast, high resolution accessories for your microscope, you can also see these membranes, but not as dramatically as with the simple addition of a drop of inexpensive ink!
Methylene blue is another stain which every biological microscopist should have on hand. Fortunately, some of the small biological supply houses which you can find online sell small amounts of powdered stains to amateurs and others offer small quantities of stain in liquid form, ready for use which is often an advantage. Methylene blue has a wide range of applications, in part, because it is a polychrome dye; that is, it is never a completely “pure” stain; it is always a stain which contains small amounts of other red, blue or purple dyes, so from batch to batch you may get slightly different results. However, one of the things that makes Methylene Blue so much fun to work with is its versatility. Like Neutral Red, Methylene Blue can function as a vital stain, but in higher concentrations, it is lethal and less predictable. It is a nuclear stain, can be used as a contrast stain, and in certain procedures, can be valuable for examining nerve cells. In micro-dissections of organisms, such as leeches or small marine polychaetes, a bit of the stain dropped onto the specimen can enhance the visibility of a variety of structures.
I am always on the lookout for calcareous structures in everything from protozoa (such as the “skeletal” plates in the rumen ciliate Diiplodinium eucaudatum) to the plates embedded in the cellulose tunic of the ascidian, Styela plicata, in short, virtually the entire range of invertebrates. Alizarine Red S is a dye that has a special affinity for calcareous material and I’m sure that many of you have seen specimens of fingerling fish where the tissue has been cleared and the skeleton stained a distinctive red by the use of this dye. If you are interested in calcareous material in invertebrates, this is definitely a stain worth experimenting with.
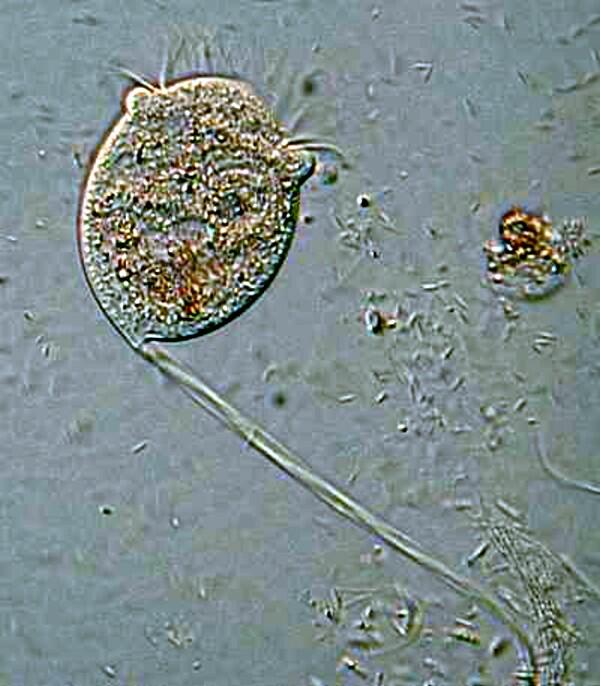
A long term puzzle regarding Paramecia is the function of trichocysts. There are extremely minute thread-like structures coiled in tiny capsules which are located just under the surface of the membrane or pellicle and which occur in enormous numbers. If you observe a specimen which has been strongly flattened by a cover glass, you can see these little structures line up along the edge of the pellicle. Interestingly, when the cell lyses (breaks open), the trichocysts are rarely discharged. However, if you wish to observe their discharge, a drop of dilute tannic or acetic acid will produce spectacular results. If you don’t have access to these, you can either brew up a strong pot of tea which will produce a solution with tannic acid in it or you can use some vinegar which is basically diluted acetic acid. Some investigators recommend using a drop of blue ink followed by a drop of red ink or vice versa, I can never remember which, but I’m sure you’ll quickly figure it out. The alleged advantage of this method is that it stains the trichocysts and it does, but I have found that it also tends to create a rather muddy cloud in the water that can obscure detail. If you want to stain the trichocysts, then trying either a dilute solution of aceto-carmine or tannic acid followed by a weak solution of stain such as methylene blue is preferable.
The mystery about trichocysts is that they don’t seem to do anything. Think of a Paramecium as a kind of 3-dimensional micro-submarine with batteries of these structures embedded all around the surface of the pellicle Now, just think, in an organism this size the production and alignment of these structures in such enormous numbers requires a significant amount of energy. Even more remarkable, however, is the energy involved in the discharge of these odd “threads” as you will discover once you give some specimens the “acid” treatment or as you can see in the photographs below.
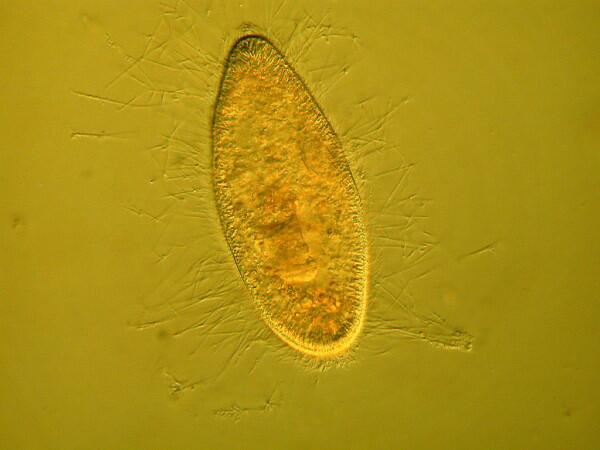
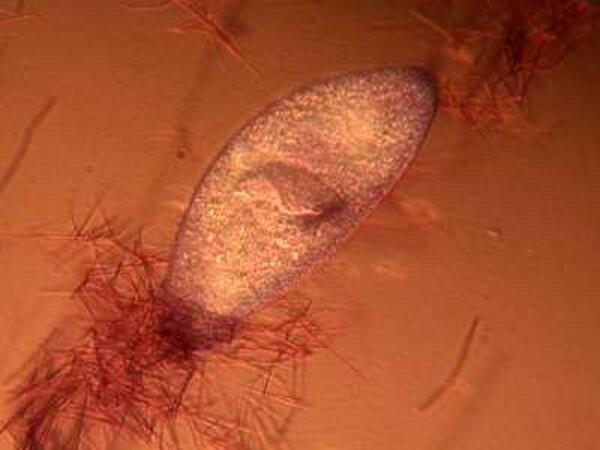
Here it is evident that the trichocysts are ejected with such force that a small cloud of them may be created around the organisms virtually obscuring the Paramecium and in some cases, there may be a scattering of trichocysts at a distance 2 or 3 times the length of the organism! And yet, this seems to be a completely useless tactic at a very high energy cost. This barrage doesn’t deter predators; it is not used for food capture, so trichocysts seem to have no utility, which, in the long run, doesn’t make biological sense. Some biologists think that they are vestigial organelles which did at one time have a defensive utility. It is well know that other ciliates, such as Dileptus, have toxicysts located around the cytostome and they contain a drop of venom to paralyze their prey and possibly deter predators and from my observations this seems a quite effective mechanism. This, however, simply compounds the puzzle since Paramecium is much more frequently and widely encountered than Dileptus. Electron photomicrographs show the trichocysts of Paramecia do indeed have a pointed tip and look as though they might once have served as a weapon of detergence. [See Kudo, Protozoology, 5th edition]. If they are useless, why haven’t they disappeared? Remember we have been observing Paramecia for only a little over 200 years and sometimes evolution is very slow in getting rid of unnecessary parts and/or behaviors.
Another household reagent that can be very useful in your lab is iodine which is readily obtainable either a s a tincture in alcohol or in aqueous solution. This can be valuable for bacteria, protozoa, and for botanical material in detecting starch compounds. Sometimes with certain ciliates, iodine enhances surface structure and one can get glimpses of features not ordinarily easily observed.
If you have organisms that will undergo drying without massive distortion, such as Paramecia, you can add a drop of Nigrosin to a drop of rich sample on a slide and let it dry. Usually some of the organisms will dry in a fashion that reveals considerable detail of the pellicle. One can also get good results with some hypotrichs. How does one determine with which organisms this technique will be effective? Trial and error. Micscape and other online microscopy sites could provide a wonderful service by compiling lists of this sort from reports by contributors. Think how convenient it would be to have an ever-growing list of ciliates suitable for examination of Nigrosin and another list of protozoa which reveal good detail by treatment with iodine, etc; the possibilities are endless-so contribute both information and money to Micscape to help such things become a reality. Micscape is a marvelous resource and it costs a considerable amount and furthermore it takes time, energy, and expertise to make it work. Actually, I’m just making this pitch so that Dave Walker and Mol Smith will triple my salary for my articles, but remember that 3 times nothing still isn’t very much, but the joy I get from having a tolerant audience of readers is incalculably wonderful.
This essay has gotten too long, but I still have some other bits and pieces about micro-technique and collecting, so I’ll save them for a future issue.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .