|
Spring and Summer Collecting: A Repeat Visit by Richard L. Howey, Wyoming, USA |
In the spring, an old man’s fancy lightly turns to thoughts of ponds and streams (or least, they do if he is an aquatic microscopist). [With apologies to Alfred Lord Tennyson]
These days, in my dotage, I am no longer quite up to wading through mud and water weeds and tend to rely upon younger friends or students to help me by collecting samples. I give them a set of instructions about preparation: mosquito repellent, sun screen, a couple of small fine-mesh nets, 4 ounce plastic bottles, a good pocket knife, a magnifier, and a knapsack. I also urge them to make relatively brief trips–not more than 3 or 4 hours using a car. The samples jar should be filled about 2/3 with pond or stream water to allow for proper oxygenation and then after sweeping a net vigorously, the resulting capture can be placed in the jar. Sometimes, a small amount of pond weed can be added to advantage as well. However, it’s crucial not to overload the jar with material and it must be kept out of the sun.
It is also important to note the location of the source of the material and the general conditions. As the conditions change, so will the population of organisms. Here at high altitudes, the water tends to be quite cool, even cold, in early spring when the snow melt from the mountains is still feeding the ponds, rivers, lakes, and streams. For example, there is one sizeable lake where, in early spring, I often find specimens of an intriguing group of protozoa, tintinnids. However, by early summer, they are gone. Furthermore, these conditions can change significantly from year to year. However, up here, it is clear that the populations change very obviously as one moves from spring to summer when the water warms substantially and there is a high rate of evaporation, especially for temporary ponds and small streams.
Some animals, beavers, in particular, can change the waterscape of an area in a short time. Some ponds that I used to delight in for collecting have now disappeared completely as a consequence of their activity. One pool where for over 10 years, I would, in the late spring, find quantities of Volvox is now completely overgrown.
What can one expect to find when one goes micro-hunting? Well, obviously, that will depend upon your location and factors such as rainfall and snowmelt. So, I will be mentioning organisms from this area, but many of them will be cosmopolitan, so some version of them will exist in many different places in the world.
It is the rare aquatic environment where there are no protozoa, so when you get your samples back to the lab, these are definitely to be anticipated. I almost always examine the samples immediately, looking for organisms that might need to be separated out either because they are predators on other things in the sample or because I may want to try to culture them. Then after a day or two, after the samples have had a chance to settle and stabilize a bit, I check them again to see what has “hatched” or excysted. And, I am often surprised at what may turn up after a day or even a week or more. We always need to remind ourselves that these jars are unnatural environments and only remotely like those from which we snatched them. There will be temperature changes and changes in the water chemistry as new organisms make an appearance and others die off. Also, if you decide to add a bit of food, to provide bacterial growth for other organisms, then you have altered the environment is a fairly radical manner. A boiled wheat grain or two can produce marvelous results or produce a foul mess. There are places here where samples can turn smelly very quickly as a consequence of sulfur bacteria which thrive in certain muddy bottoms.
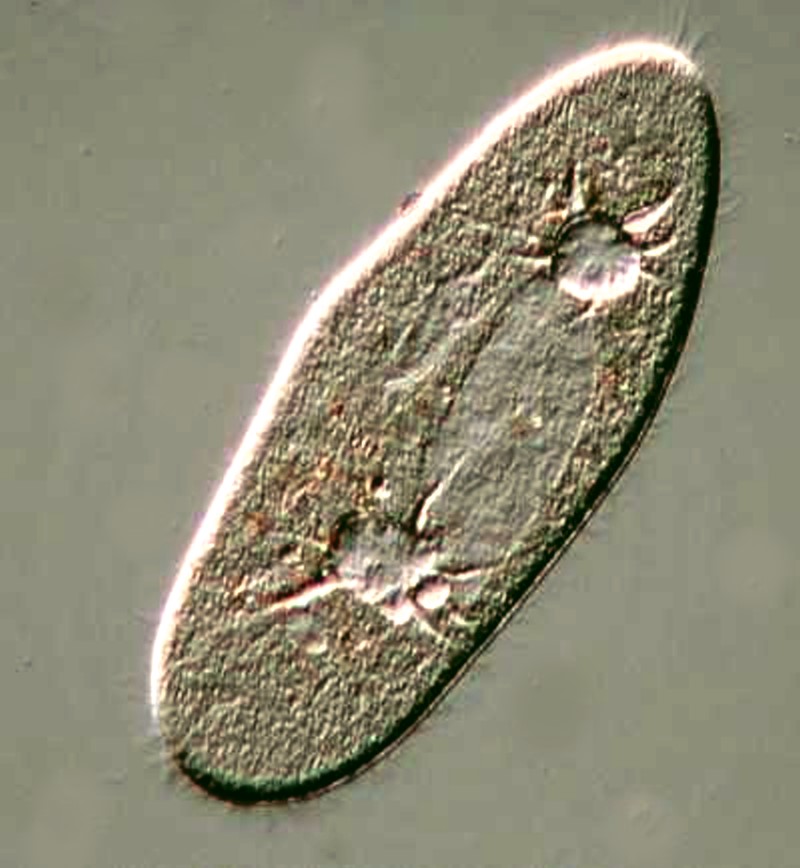
Back to protozoa. A very likely candidate to make an appearance is, of course, Paramecium. Because it is quite common, it, unfortunately, is taken for granted, but as I have tried to demonstrate elsewhere, it is a very remarkable beastie. Below are a few images to show you some of the more interesting features.

Here we can see the large ovoid macronucleus and the 2 contractile vacuoles with the canals that feed into them.
By adding a drop of Harris’s Hematoxylin to a drop of rich culture, one can sometimes reveal paramecia in various states of development. In this case below, we have a nice view of the chromosomes during a state of division.
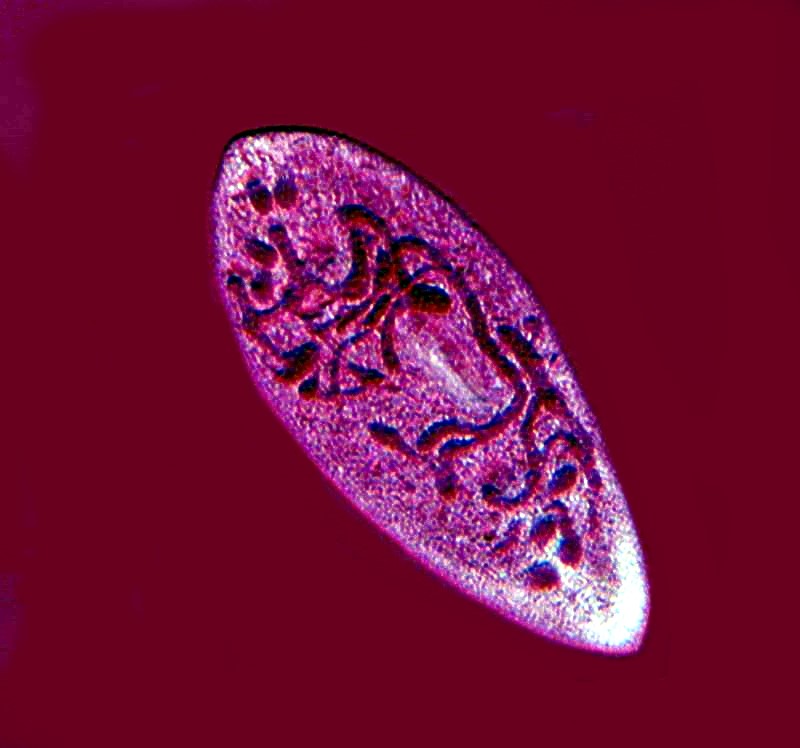
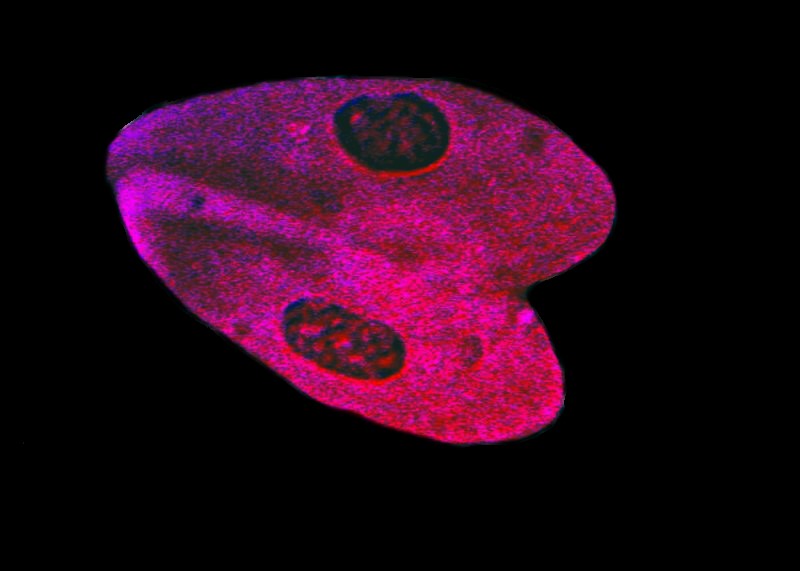
And, using the same stain, we find a pair of specimens which are conjugating.
One of the properties of paramecia that makes them especially desirable to experiment with is their hardiness. They tolerate a variety of techniques without radical lysis (coming apart) including drying. Using a drop of 1% Nigrosin and letting it dry, reveals the structure of the pellicle.

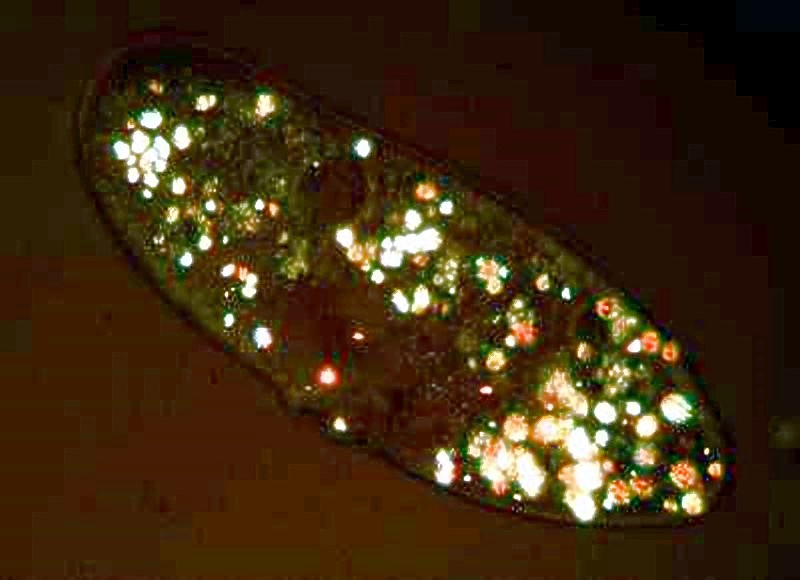
Notice the orange-colored particles scattered around in the organism; these are crystals of calcium oxalate. If we place a Paramecium in polarized light, we can see them readily. If the specimen is still alive, it will appear like a tiny motorized boat gliding through the water at night.
Paramecia have tiny hair-like “harpoons”, called trichocysts enclosed in tiny capsules and distributed all around the organism just under the pellicle. A tiny drop of very weak acid or a drop of red ink can cause them to discharge. Their function is still unknown. The first image shows a very small discharge at the posterior (upper) end. The second shows an explosive discharge and gives an idea of the very considerable force involved in their release.
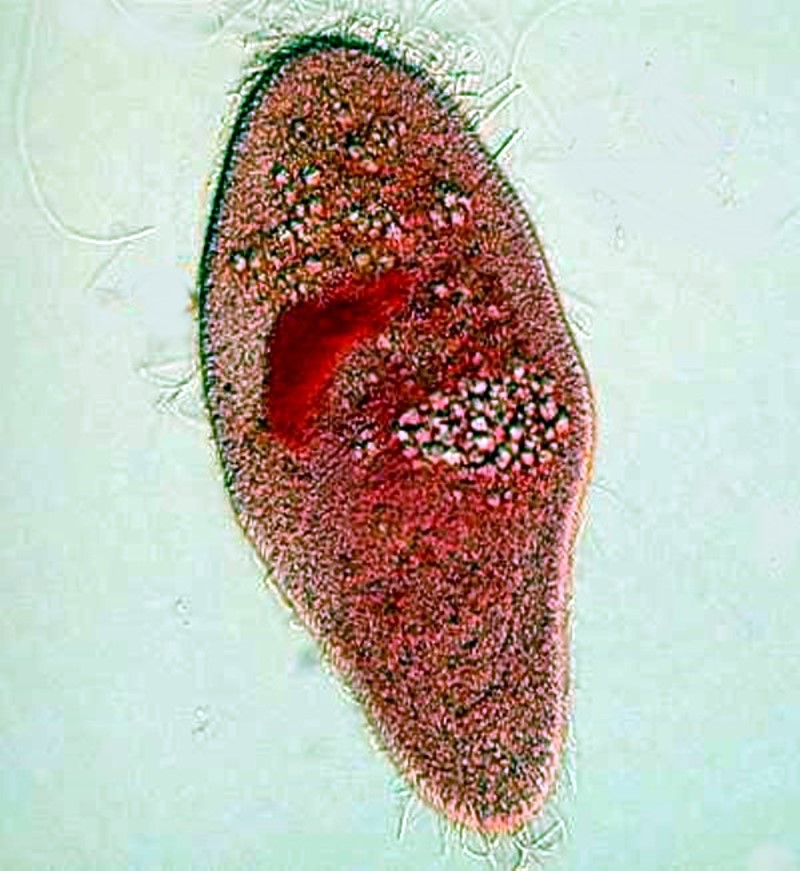
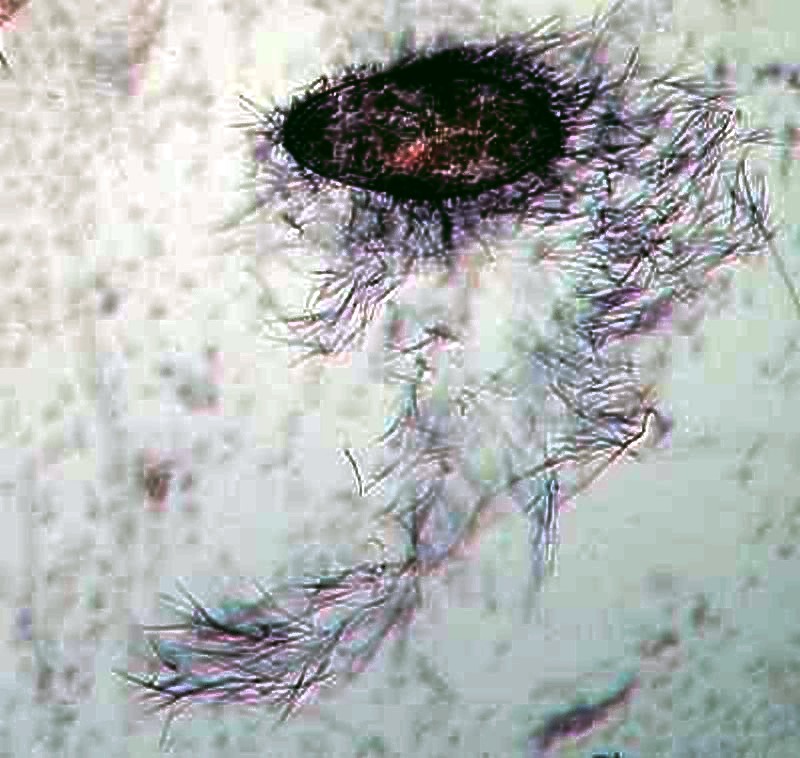
One thing that this tells us is that if we go collecting and, in the unlikely event, find only paramecia, there is still much to discover and learn.
Let’s look at a few other examples of protozoa before we turn to other critters that we might anticipate finding.
Some ciliates have been discovered to possess an elaborate system of fibrils called “the silver-line system” which coordinates the movement of the cilia. This is a specimen of Blepharisma, a fascinating ciliate, some species of which are pink and this creature sometimes turns cannibalistic and produces “cannibal giants” which will have food vacuoles containing other ingested Blepharisma and, as a consequence, take on a deep red color. This specimen was treated with the elaborate technique of protargol silver staining.
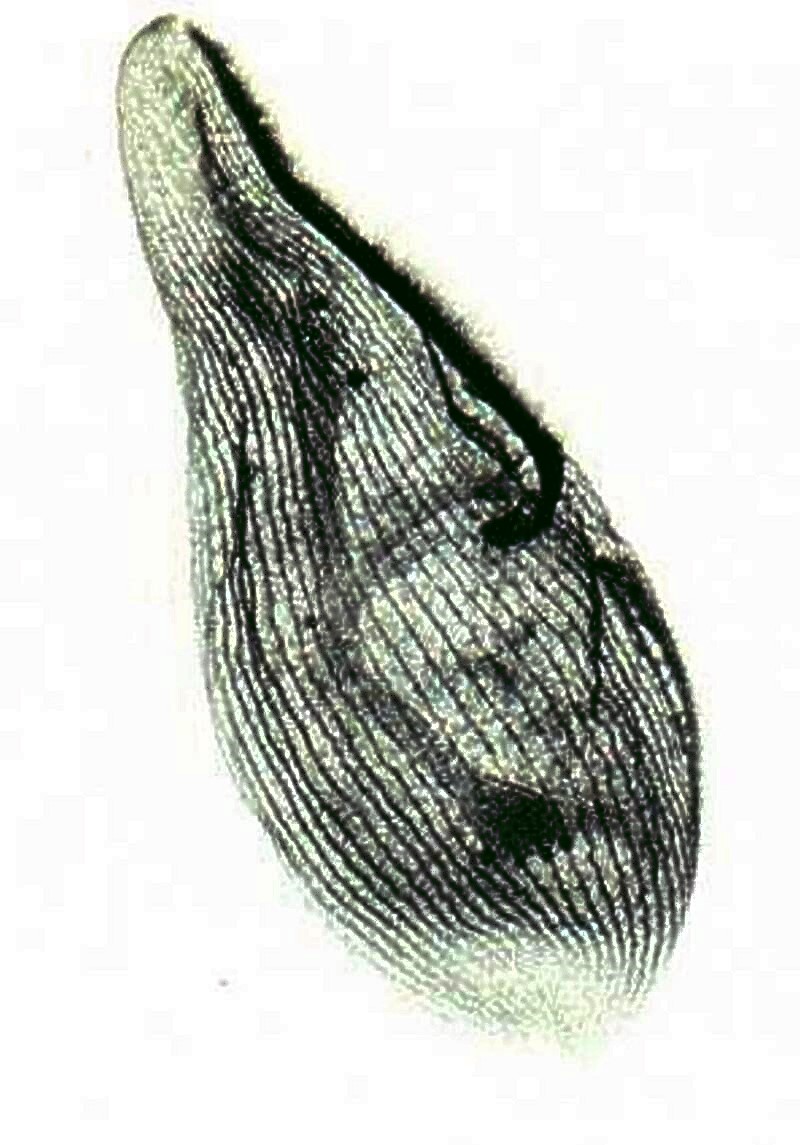
Another organism stained to show its silver-line system is Euplotes and you can see the result below.

Euplotes is a hypotrich and has bundles of fused cilia called cirri and they often crawl along on these along a substrate. Using the stain Nigrosin, we can clearly see the cirri and also the “sculpting” of the pellicle.

If you let your samples settle for a couple of days and then examine the sediment, you may well find some amoebae. Amoeba proteus, because it is easy to maintain in culture, is often provided by biological supply houses; but, interestingly, in nature it’s relatively uncommon. I have found it only twice and, most recently, in a large lake at about 6,000 feet.
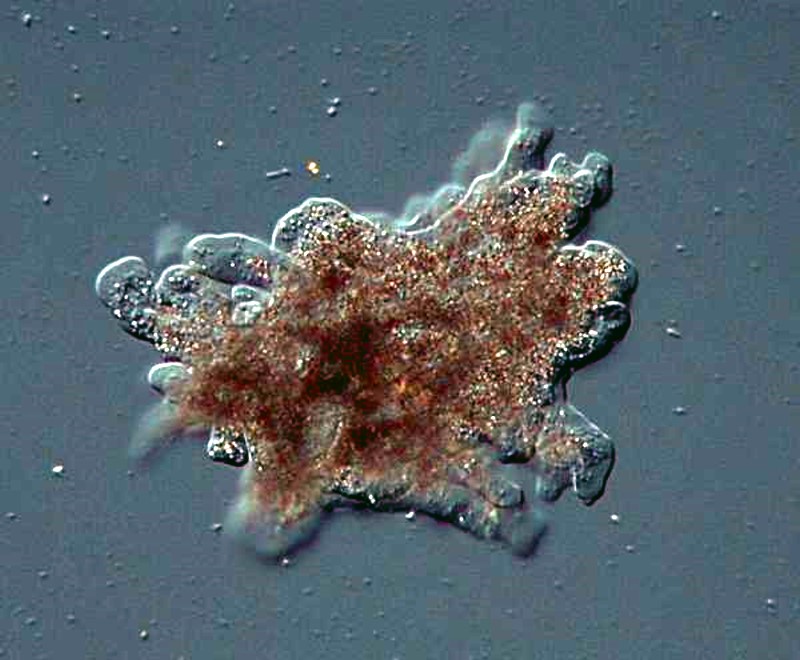
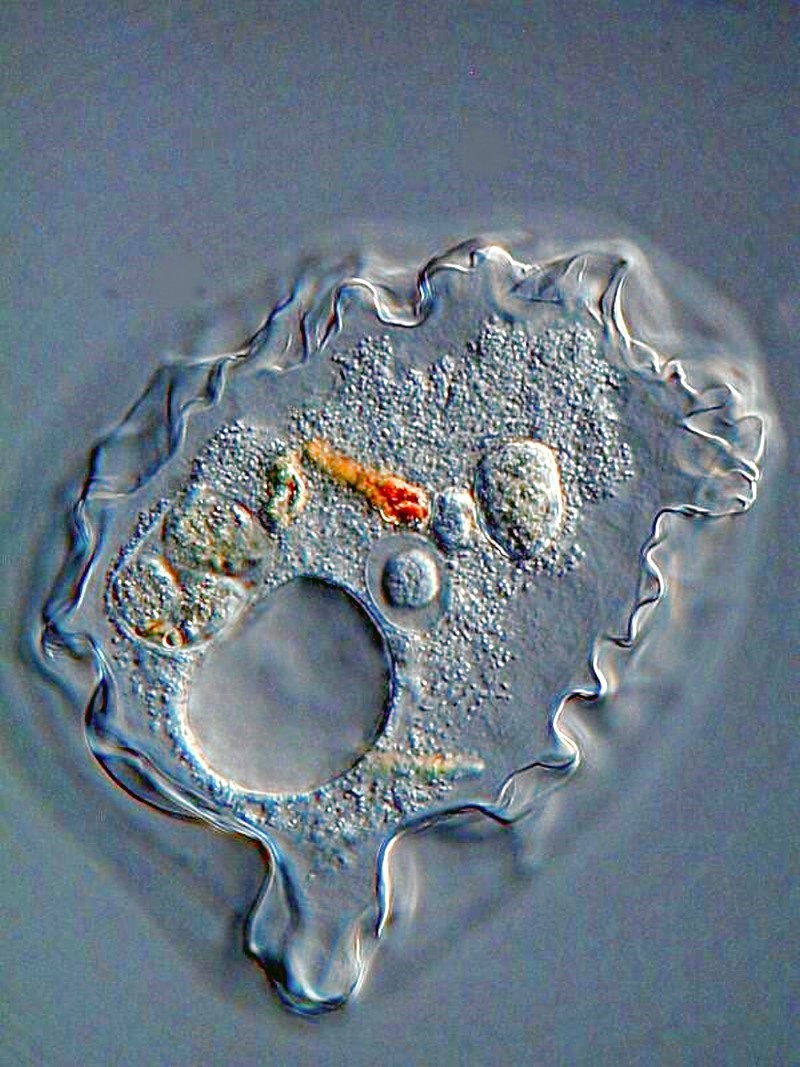
However, small amoebae are often quite abundant and some are quite entertaining to observe, like this ambitious little guy trying to ingest a very long algal filament.
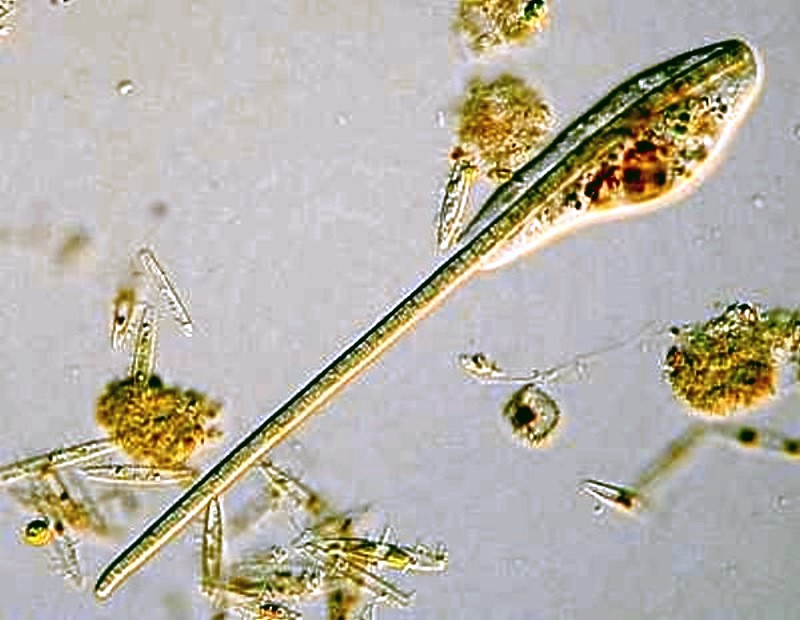
We can learn from small amoebae to set ourselves large goals and be persistent; just consider the two examples below. The first is a small amoeba in the process of engulfing a sizeable rotifer–talk about stretching out one’s resources.
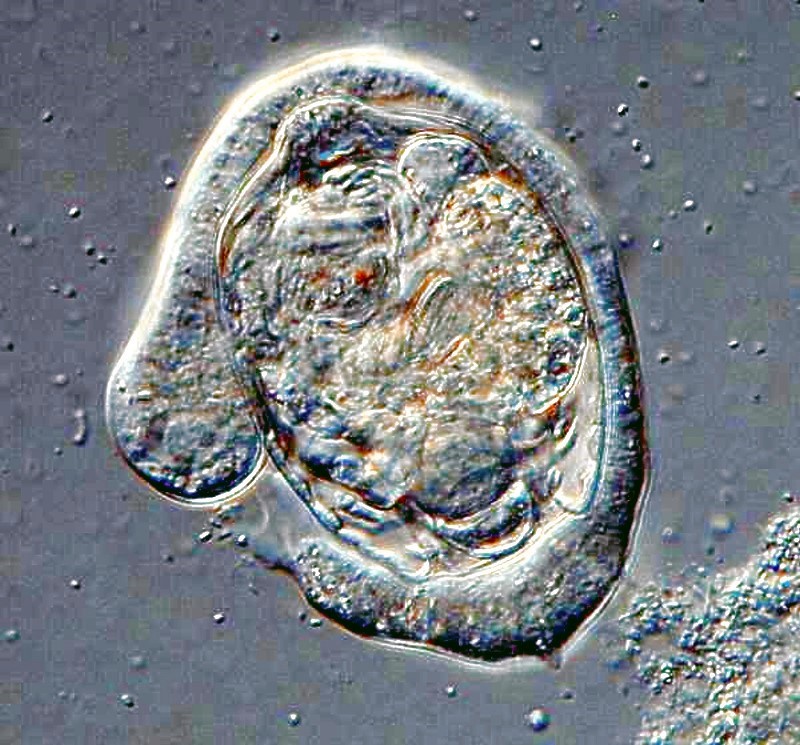
Or, another one trying to tackle a Euplotes and not having quite as much luck.
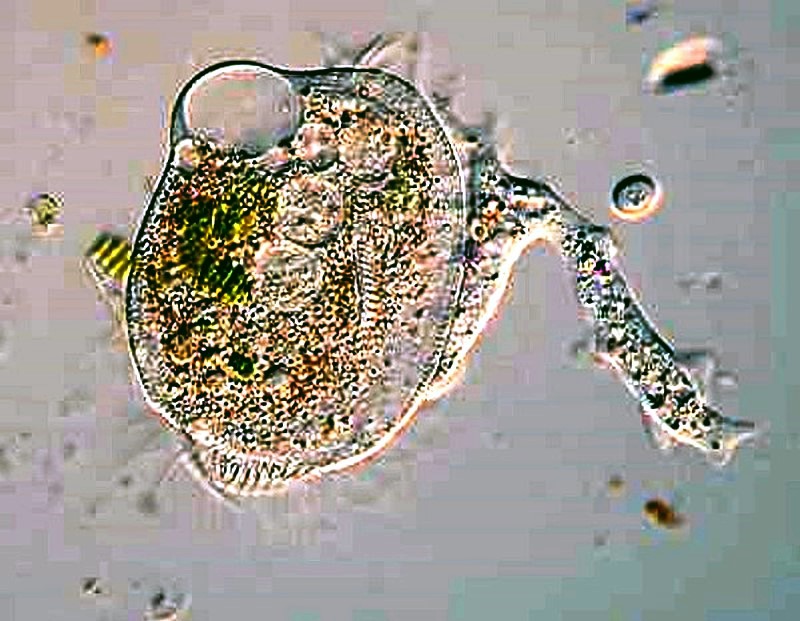
Now, if you get really lucky, you might find some Thecamoeba which are also avid algae eaters.
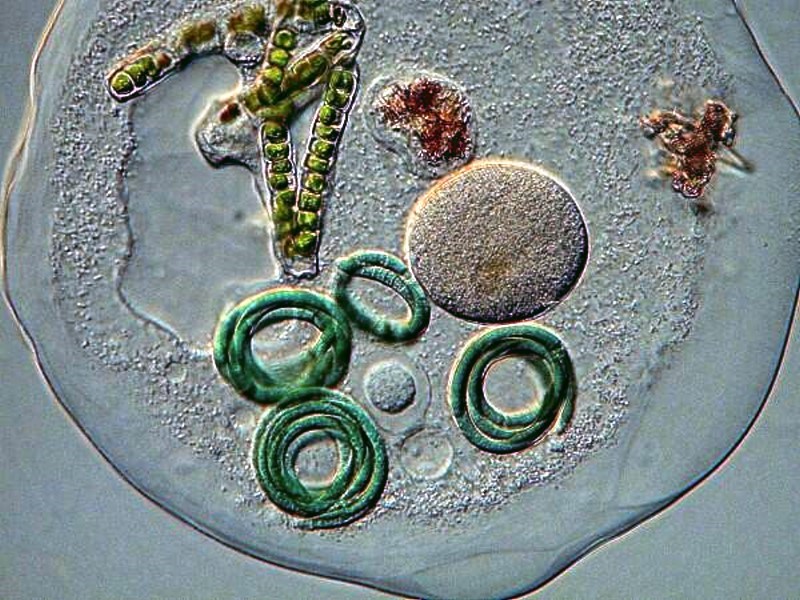
It is very likely that you will encounter some desmids and diatoms. Here is a very nice desmid called Closterium.

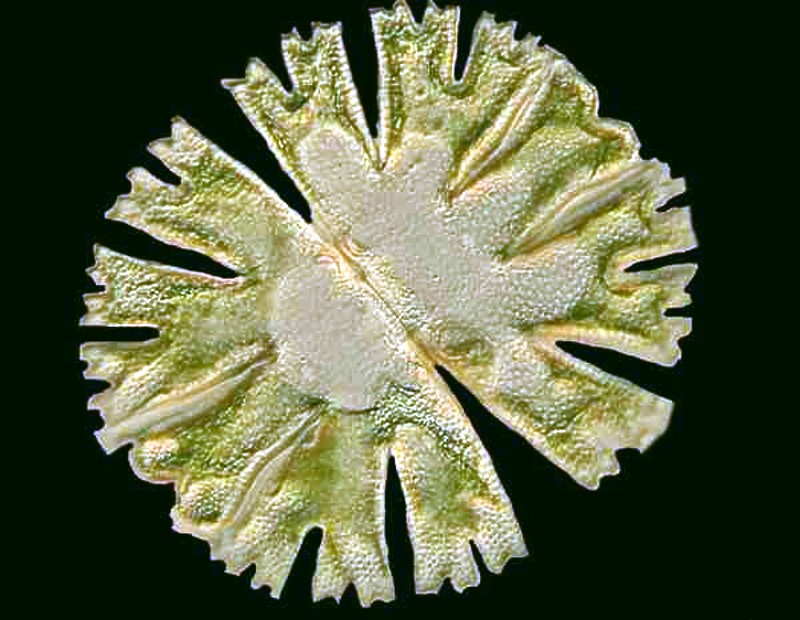
And then, there is the splendid Micrasterias.
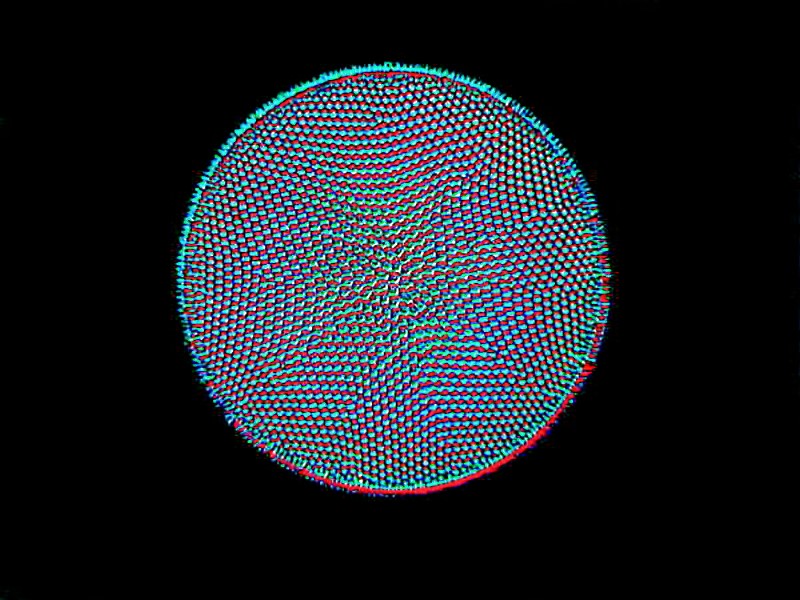
My experience has been that desmids rarely occur in abundance, but they are well worth spending time looking for. Diatoms, on the other hand, occur in great variety and in a wide range of habitats and sometimes in great abundance. I’ll show you examples of 3 major types. The first is a centric diatom.
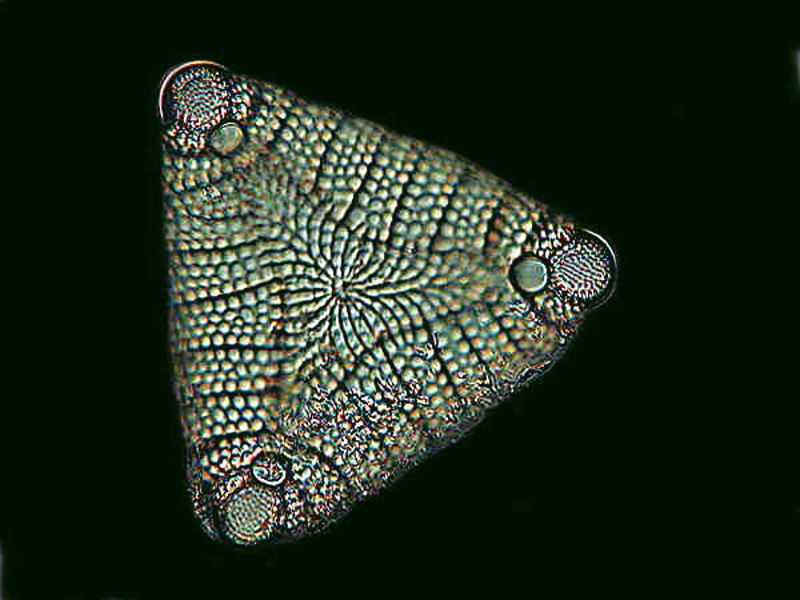
The next two are examples of pennate diatoms which are bilaterally symmetrical. The first is Cymbella and the second is Etogonia.

There is a very common filamentous alga which you are quite likely to encounter, namely, Spirogyra which has all kinds of interesting characteristics and behaviors including conjugation.
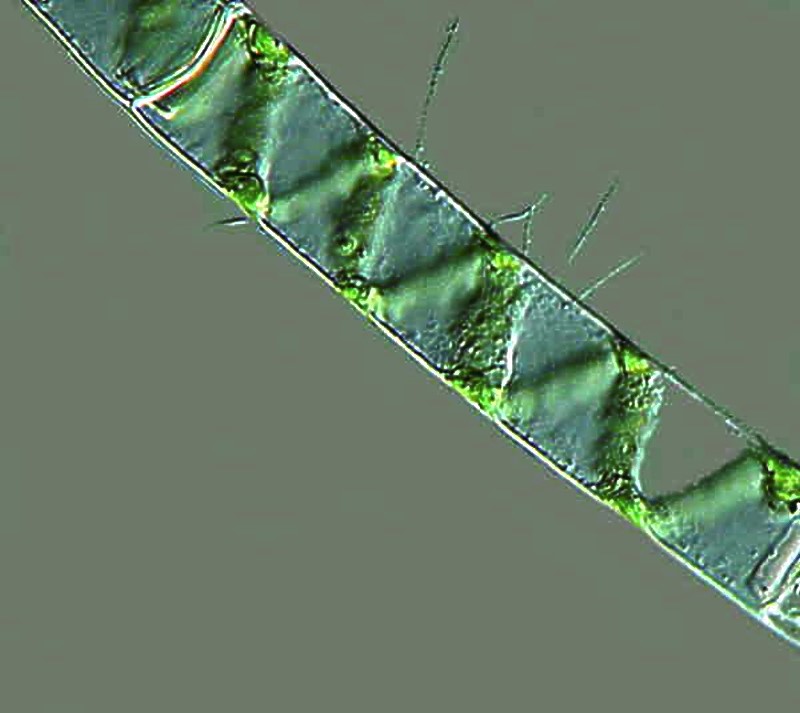
Now, let’s take quick look at a couple of rotifers. This is a group of organisms which shows great diversity both in form and behavior. They are multicellular and are highly complex.
This first one is Kellicottia and again, I collected this from a large high altitude lake.

This next rotifer is Keratella and I added a drop of the stain Basic Fuchsin in order to show the sculpting on the lorica, the semi-hard shell which forms the outer surface.
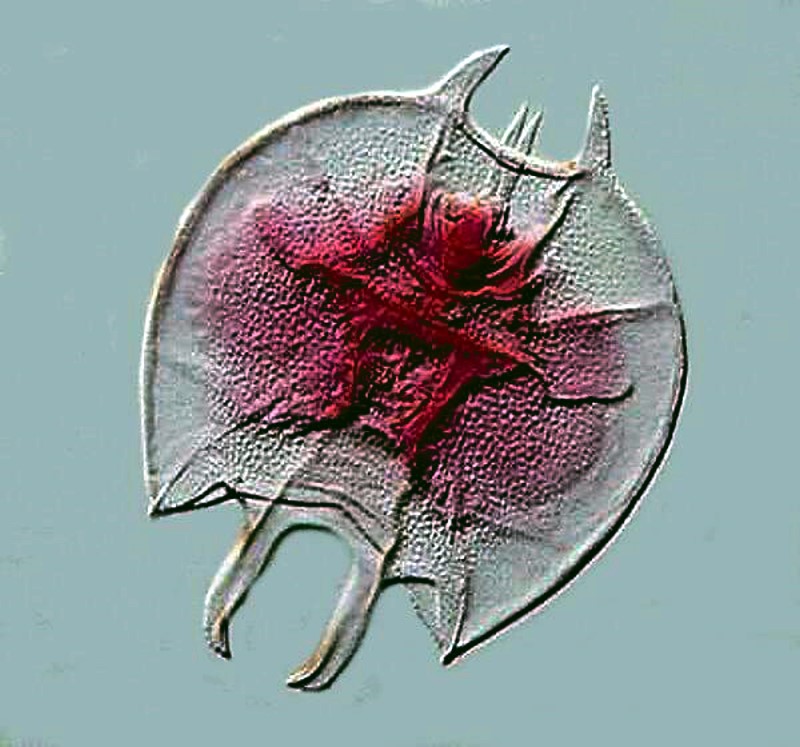
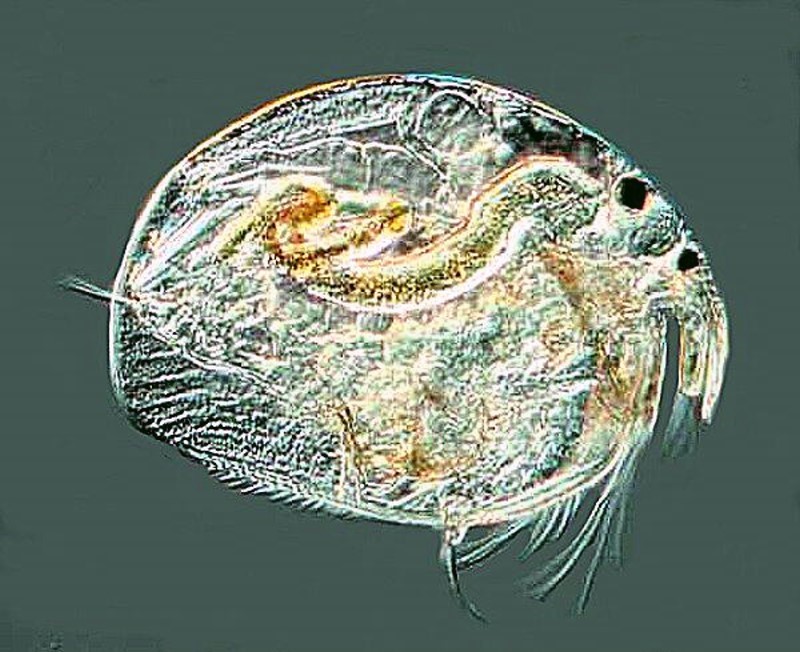
And finally an image of a cladoceran or “water flea”. You might encounter a variety of these including Daphnia which is larger. These organisms sometimes occur in astonishing numbers.
There are many other possibilities, tadpole shrimp, rhabdocoel worms, nematodes, bryozoa, tardigrades, and an enormous variety of insect larva, but it’s time to go get your net and bottles and start collecting and reveling in the joy of discovery.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the July 2016 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .