|
|
A Gallery of Sulphur Photomicrographs ( using polarized light )
by Brian Johnston (Canada) |
|
|
A Gallery of Sulphur Photomicrographs ( using polarized light )
by Brian Johnston (Canada) |
The element sulphur is often remembered by chemistry students as "a yellow solid with an unpleasant smell". Although this is undoubtedly true, sulphur is an extraordinary substance which is much used in industry. Products as diverse as food preservatives and explosives contain sulphur as part of their molecular structure.
Sulphur is a non-metal which exists in different crystal structures known as allotropes. The stable form at room temperature is called rhombic sulphur, and when this is heated slowly above about 95șC, it transforms into monoclinic sulphur. Both forms are crystals made of S8 molecules like the one shown below. (Visualization by 'Hyperchem' software.)
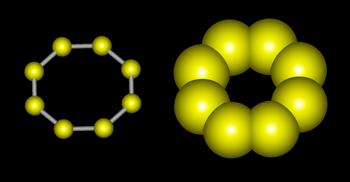

In the two forms of sulphur mentioned above, the molecules are identical. The only difference between the rhombic and monoclinic types is the arrangement of the molecules in space.
The slides used in this article were prepared by melting a very small quantity of powdered (sublimed) sulphur which had been placed between slide and coverglass. The heat source was an alcohol lamp. CAUTION! Sulphur melts at 113șC and the heat of the alcohol lamp may cause the molten sulphur to ignite and burn with an almost invisible blue flame. This is particularly likely if any small grains of the solid were left near the edge of the slide where they might contact the flame. The burning sulphur reacts with the oxygen in the air to produce the very poisonous gas sulphur dioxide. Although this is a rare occurrence, I always keep a damp paper towel nearby to smother the flame. Working in well ventilated surroundings is also important. As always when melting chemical substances, heat should be applied very gently. This is particularly true for sulphur for reasons that I will mention later.
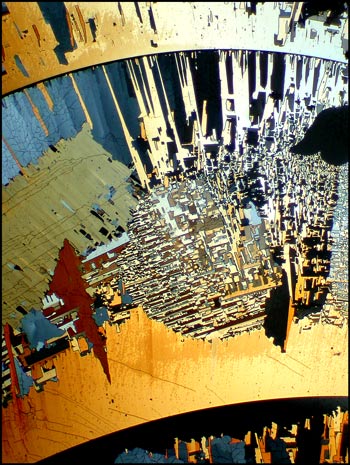
What the prepared crystals will look like when illuminated by polarized light under the microscope, depends on many factors. The most important thing to keep in mind is that there is a limited time to photograph the crystals. For example, consider the first image in the article. After from several hours to a day (depending on the ambient temperature), the field of view is transformed into a less interesting, more amorphous form. I suspect that when the melt first crystallizes, it is of the monoclinic (high temperature) form. As the temperature cools, it changes into the rhombic (low temperature) form. This process takes some time because it occurs in the solid state. The result of this transformation can be seen in the image below. Most sulphur fields look like this eventually, and this means that it is not possible to make permanent mounts of particularly interesting formations.

If the thickness of the sulphur crystal film between slide and coverglass is ideal, the structures will be very colourful. Notice the characteristic curves in the images below. (The polarizer and analyser are adjusted to give extinction (black background) in all of the images.) The bottom left image was produced using an additional l/4 plate.



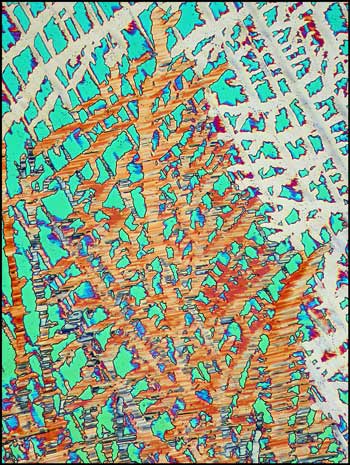
During the solidification of some melts, I apply pressure to the centre of the coverslip in order to provide a smoothly changing thickness of the crystal layer. (Unless you want to burn a finger, I suggest using an old glove for protection. Better still, use something like a pencil eraser.) If I am lucky - (Yes, chance plays a large part!) - fields like the ones below will appear. To me, they look like crazy ramps in the style of M. C. Escher. These fields tend always to be in shades of grey or brown.




I mentioned earlier in the article the importance of gentle heating when melting the sulphur. If this is not done, circular bubbles will be visible in the image and these tend to spoil the result. Unfortunately, if the slide is remelted, the chance of these bubbles appearing increases tremendously. Because of this, I seldom remelt sulphur slides more than once.
For some unknown reason, some of these Escher type fields contain brightly coloured inclusions. The image on the right below shows a magnified portion of the one on the left.


Other examples of this phenomenon are shown below.




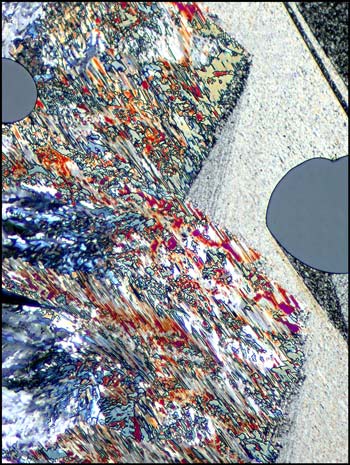
The examples above tend to appear in the thinnest areas of the crystal layer. As the thickness increases, lines become broader and tend to look more tubular. Some colour begins to appear. The example images below demonstrate this.



Sulphur is, in my opinion, a very photogenic subject. After working with it for years, I can almost predict the general form that I will see under the microscope. Fortunately however, there are many surprises. In the images below, lambda and lambda/4 plates were used to change the background colour.

So far, in my series of articles on interesting polarized light subjects, all of my choices have been compounds. Sulphur is the first that is an element. It is probably the only non-metallic element that it is practical to study under the polarizing microscope. Metals, of course, are studied by metallurgists using specially designed incident light microscopes. (There are several articles on this subject in the Micscape archive.)
The photomicrographs in the article were taken with a Nikon Coolpix 4500 connected to a Leitz SM-Pol microscope.
All comments to the author Brian Johnston are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy
UK web
site at Microscopy-UK