|
A Micro-Rothko Gallery of Crystals by Richard L. Howey, Wyoming, USA |
Who would imagine that taking photomicrographs of Magnesium chloride crystals would lead into an adventure in the world of abstract expressionist art. For some months, I have been experimenting with a variety of substances to investigate interesting types of crystals which will produce colorful and intriguing structural results with polarized light. I found a small dropper bottle of a 33% solution of Magnesium chloride hidden at the back of a shelf. I’m not sure when or why I ever mixed it up, but I suspect that it’s at least a decade old and that I was trying it out to see if it would work as an immobilizing agent for aquatic invertebrates in the fashion of Magnesium sulfate which, by the way, is a very useful substance of the amateur microscopist.
I have a vague recollection of having tried to observe crystals of Magnesium chloride before and apparently I was not especially impressed. This may be partially attributable to the fact that Magnesium chloride is exasperatingly hydrophilic (hygroscopic)—in other words, I put a drop on a slide, wait a few days or weeks and then find that, even in the very dry climate in which I live, although there are indeed some crystals, there is still unevaporated liquid around them.
Aaaargh!
I even tried making some quasi-melts by heating the solution, but as soon as it cools, the crystals start to absorb water out of the atmosphere again. To make true melts, one needs to place a bit of the dry Magnesium chloride on a slide and then heat it using a slide-warming tray. [CAUTION: Here again, even with what seems like a safe enough substance under ordinary circumstances, one has to be careful not to heat it to its decomposition temperature as it will produce corrosive hydrochloric acid fumes and at 300 C (572 F), it will produce toxic chlorine gas.] However, I have learned that waiting a few days and letting the slides sit at room temperature is well worthwhile and the small amount of liquid around the individual crystals is rarely a problem if one takes a bit of care with positioning and lighting when capturing the image. Any slight shadowing or reflection can be removed when processing the image on the computer. Most of the crystals are relatively small and I usually photograph them at magnifications in the range of 100x to 300x.
If the polars are fully crossed then, especially at higher magnifications, it becomes difficult or impossible to see the crystal on the camera’s view screen even at full illumination. Rotate the polarizer until you get a clear image and then center it in the frame and adjust focus. Sometimes such partially polarized images give very pleasing results in and of themselves.

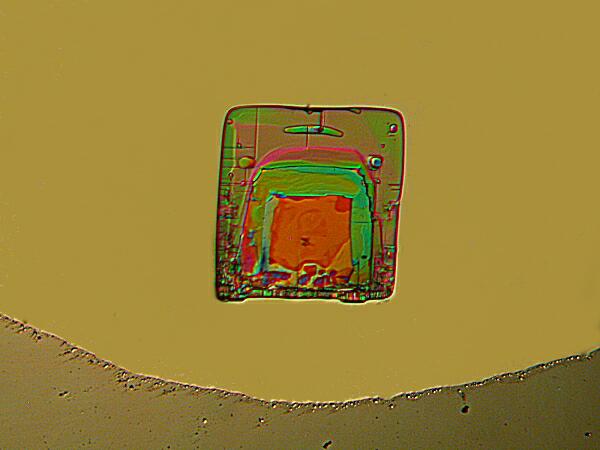
Partially Crossed Polars
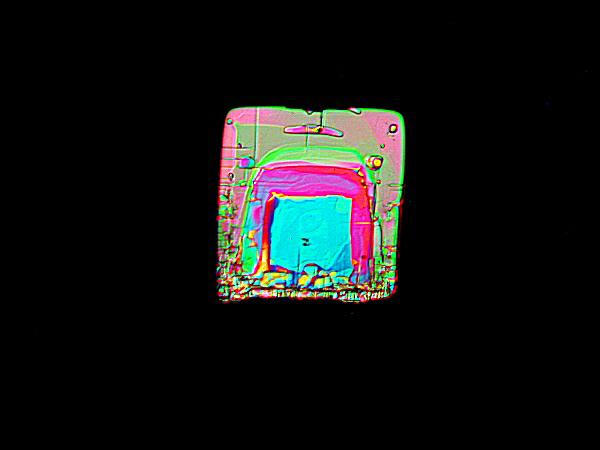
Crossed Polars
Once adjusted, one can return to fully crossed polars and, with these crystals, I recommend reducing the light intensity, capturing an image again reducing the intensity, taking another image and in some cases repeating this yet one or twice more.—Ah, the glories of the digital age! No film to worry about wasting or processing.—The reason that I recommend this procedure is that with Magnesium chloride crystals, images taken with full intensity often seem bleached of part of their color and even some structural detail, whereas some of the “darker” images have a richness and intensity that are truly impressive.
My earliest investigations of these crystals produced an echo in my ancient memory, a kind of aesthetic resonance which made certain images seem both familiar and yet alien, in the sense that the familiarity had nothing to do with the crystals. Then after a week or so, I had one of those wonderful “Eureka!” experiences—Mark Rothko. My tastes in modern art are fairly severe and uncompromising—in short, I think much of it is Dreck and consists of gimmicks and charlatanism promoted by gallery owners who are interested merely in capitalizing economically on this week’s fad. However, I have a great admiration for the genuine creative adventurers whom I regard as explorers rather like the great naturalists who venture into unknown territories. Of course much of what I appreciate is no longer regarded as “modern”, but whatever the labels, the sorts of abstract works I like are those of artists such as Klee, Kandinsky, Rothko and Miro, but not the dribbler Pollack nor the linoleum designer, Mondrian.
After 2 months of making slides of Magnesium chloride and waiting for crystals to form, I have developed a much stronger appreciation for Rothko’s work and indeed have come to understand how one could spend years exploring essentially rectangular geometric shapes while experimenting with shadings of color. Indeed, I may yet spend a number of months photographing the demonically recalcitrant Magnesium chloride crystals.
I mentioned earlier that I have been using an old solution with a 33% concentration. This afternoon, it occurred to me that I probably had a small jar of this fiendish compound still in my chemical cabinet and, sure enough, I found one. There was about an inch of crystals on the bottom and others which were clinging to the side of the jar. I removed three of them with a pair of forceps and placed them on a clean dry slide and I had to move the forceps around on the surface of the slide and tap them against the glass to try to loosen the crystals. Every place I moved the forceps produced a trail of tiny droplets. I placed the three crystals individually and was finally able to shake them free of the forceps. I put the slide under my dissecting microscope and was amazed to observe three things:
1) Almost immediately each crystal began to melt very slowly forming a pool of liquid around itself.
2) On the surface of the crystals, minute impurities and bits of dust were slowly moving about demonstrating motion on the thin liquid surface of the crystals.
3) The pool of liquid around each crystal continued very slowly to get larger.
I got curious about the solubility of these crystals and so I dug out my old copy of Lange’s Handbook of Physics and Chemistry. The formula is MgCl2-6H2O and also has the name bischofite. The crystals are described as monoclinic and deliquescent (hygroscopic). In cold water (0 C. or 32 F.), its solubility is 281 parts; in hot water (100 C. or 212 F.), its solubility is 918 parts; in 95% alcohol its solubility is 50 parts. I put 3 drops of nearly absolute alcohol on the slide and watched as the 3 crystals melted like ice cubes in a glass of tap water (or preferably, a martini). I say “nearly absolute” because the bottle had been opened and even though I capped it tightly and then sealed it with Parafilm, the higher concentrations of alcohols are also highly hygroscopic and readily absorb moisture from the atmosphere thus becoming diluted which condition is compounded by evaporation. This slide has been sitting for 3 days now and I took a toothpick and distributed the liquid into a spread of droplets across the slide. I looked at it late this afternoon—it remained a smear of small droplets with nary a single small crystal in sight.
At this point, let me present some of the crystal images that strike me as Rothkoesque. I’ll also give you a website for an exhibition of some of Rothko’s paintings which took place a few years ago at the National Gallery in Washington D.C. I’d contact them and ask them to use a few images for this article, but I’m too lazy; but perhaps, more importantly, I despise dealing with government bureaucracies and by giving you the website, you can explore some of the periods of Rothko’s work, other than the one I’m interested in here in relation to the crystals. So the URL of Rothko (who is not related to the Earl of Leichester) is :
"http://www.nga.gov/feature/rothko/classic1.html" Click on the right reddish-brown arrow at the bottom of the page to see the next painting from the classic period (even though the arrow is above the description ‘toward abstraction” and you will note that “the classical paintings” description is highlighted in reddish-brown).
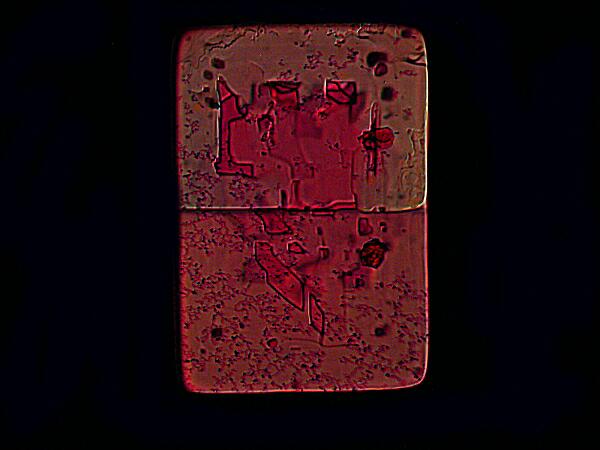
This image seems to me to be quite striking in its resemblance to Rothko’s classical period. The crystal was formed from a mixture of Magnesium chloride (hereafter MgCl2) plus a fluorochrome dye known as Rhodamine B.

This image also is reminiscent of some of the paintings of the classical period, although Rothko generally used more muted colors. This crystal was formed from a mixture of MgCl2 and a brand of clear Mecurochrome.

This image is also suggestive of Rothko in its display of dark colors and irregular forms within an essentially rectangular format. This was formed from MgCl2 and a biological stain called DAPI discussed below.
In this next section, I want to show you some examples of the results of mixing yet some other substances with Magnesium chloride as well as some additional examples with the chemicals above. Again, remember the previous cautions and, in addition, don’t mix Magnesium chloride with oxidizing agents, since again you run the risk of generating chlorine gas.
My first mixtures consisted of adding a drop of a stain to a drop of the Magnesium chloride solution, then adding a drop of 70% isopropanol (rubbing alcohol) then, using a toothpick, spreading the solution into little pools and droplets across the slide. As you have seen from the images above, Magnesium chloride can be very colorful and geometric. Nonetheless, I was curious abut how some stains and other compounds might affect the crystals both in terms of color and structure. I started with a very dilute solution of DAPI which a colleague had given to me years ago—in fact, 2 decades ago, as the label read 1984. I don’t have the proper dichroic lenses and filters to use it as a fluorescent stain and although the solution is clear and I suspected no longer active, I thought I might as well try it, since I certainly wasn’t getting any use out of it as a fluorochrome. So, a drop of each, followed with a drop of isopropanol, the smearing with the toothpick and then waiting several days. When I examined the slides, I was pleasantly surprised to find minute crystals with an interesting variety of form and color. The slides of the Magnesium chloride and isopropanol alone didn’t seem this intriguing. The DAPI slides produce a fair number of rectangles usually possessing at least one clear linear groove and the images generally have brighter colors that one finds in Rothko’s work. Sometimes the groove would effectively divide the rectangle in half while, in other instances, the groove seemed more randomly placed. There are also not uncommon cases of intersecting grooves, almost always perpendicular to one another.
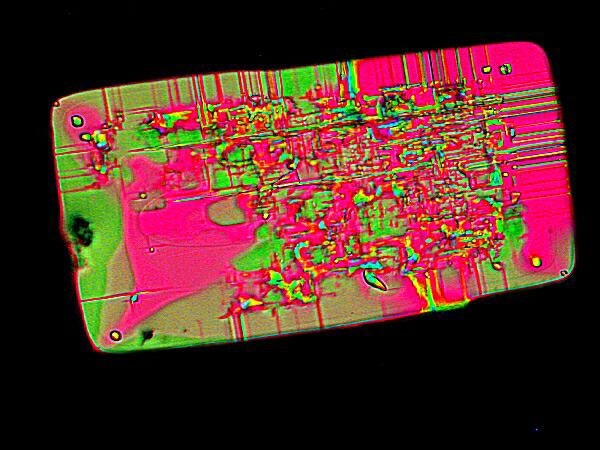
What is similar here is not only the geometric shape, but the use of pastel colors even though Rothko’s colors tend to appear more muted or bleached. This is a DAPI image.

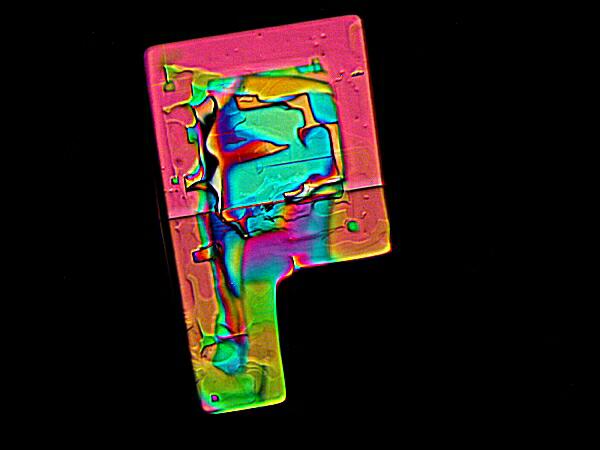
These two images show an effect that occurs fairly commonly with mixtures of MgCl2 and DAPI, namely, a rectangle with a corner “cut out”. It is also interesting to observe the remarkable degree of variation in the response of these crystals to polarization. The upper image being almost metallic in character, where as the lower image has a wide range of vivid coloration.
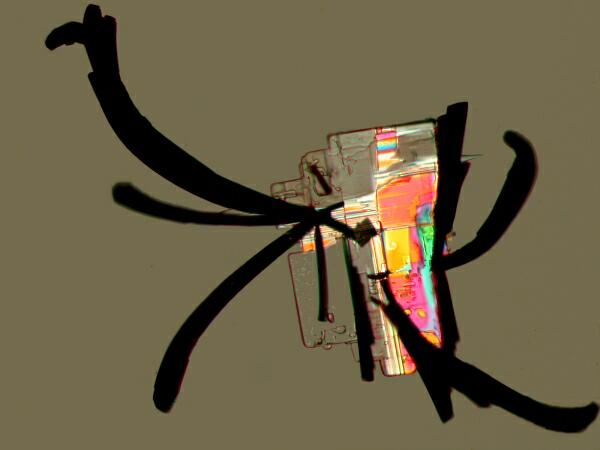
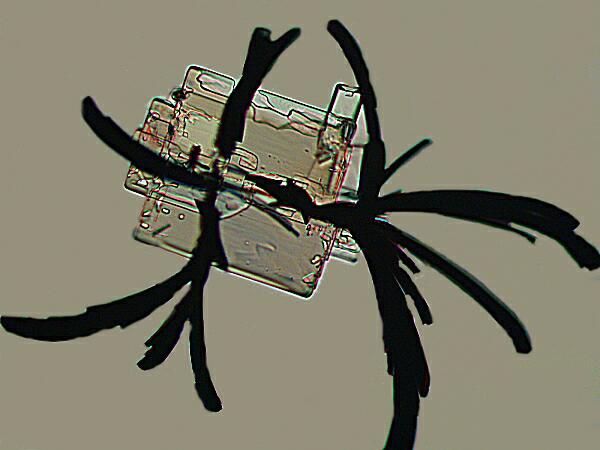
Another stain, commonly used in biological laboratories, is Methylene Blue. At first I didn’t believe the results I was getting and so I repeated the experiment several times. Each time I would get these virtually black feather-like projections attached to an essentially rectangular crystal.
As is evident from one my previous articles, I am a great fan of Vitamin C and so, naturally, I mixed some MgCl2 with Ascorbic acid (Vitamin C) and I’ll show you a few of the results below.
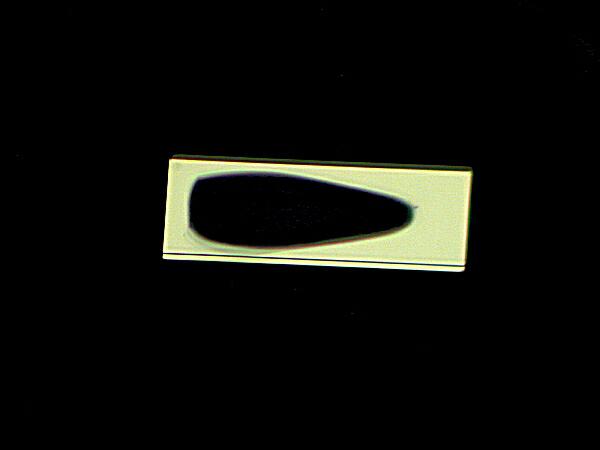
Again we find the rectangular form, but the large ovoid shape in the middle is quite unusual. It’s not particularly suggestive of Rothko, but I suspect he would have liked it.


These mixtures with Ascorbic acid are quite interesting because, although, they produce rectangular crystals, the crystals are quite small and some of them are relatively long and thin. These two images don’t remind me of Rothko, but rather of the whimsy of Paul Klee.
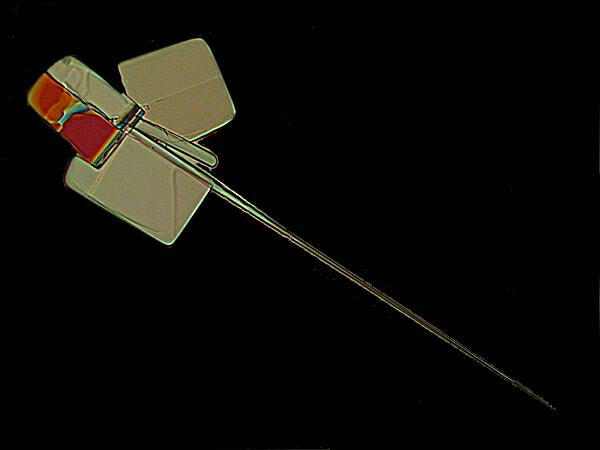
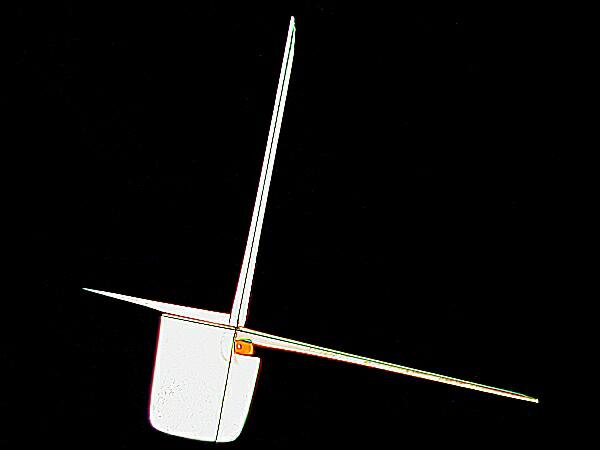
While MgCl2 does have a kind of geometric predictability, Ascorbic acid is astonishing in its variability. In the two images above, you see a marvelous combination of rectangularity with needle-like crystals which are almost certainly a result of the influence of Ascorbic acid.
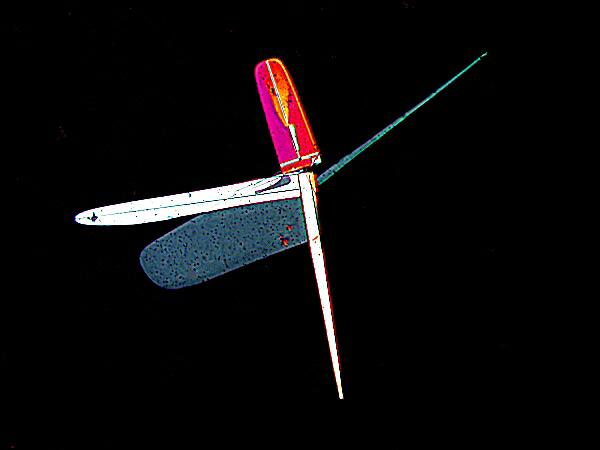
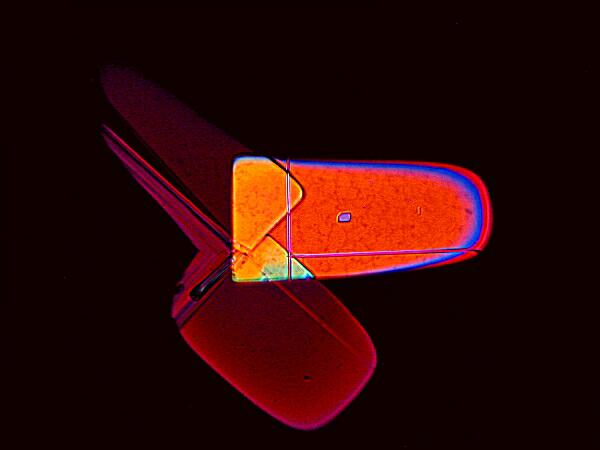
Here are two more examples of what splendid geometric variation can be achieved with the right combinations. The upper image is MgCl2 mixed with a common biological stain called Crystal Violet. The lower image is again a mixture with the stain Rhodamine B which was also used to prepare the very first image in this article. The Crystal Violet image makes me think of a high-tech satellite designed by Klee.
In concluding, I would like to present just three more images to underscore the geometric dominance of MgCl2 combinations.
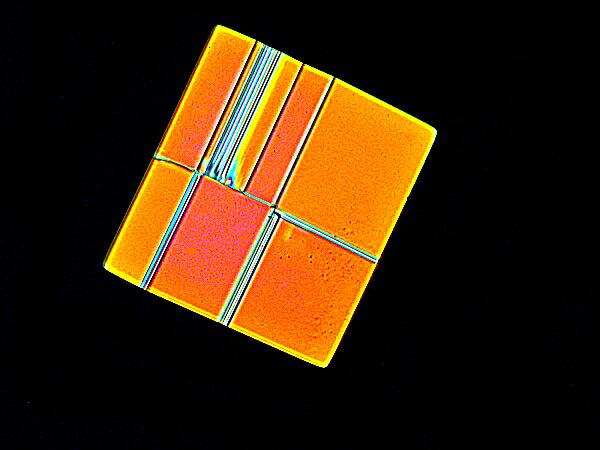
This splendidly vivid series of rectangles is the result of mixing MgCl2 with a drop of generic Alka-seltzer.
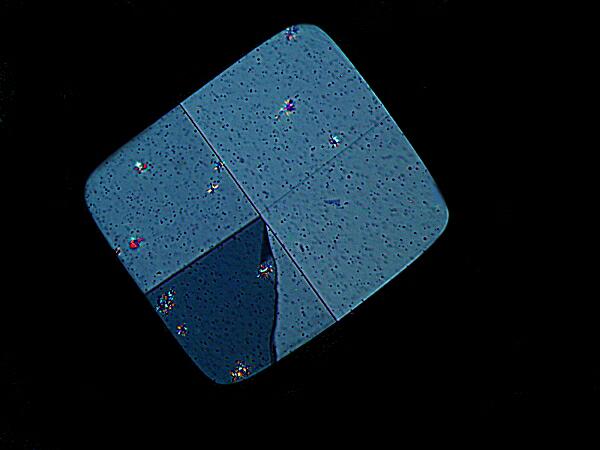
This rather subtly shaded crystal was obtained by mixing with Crystal Violet.
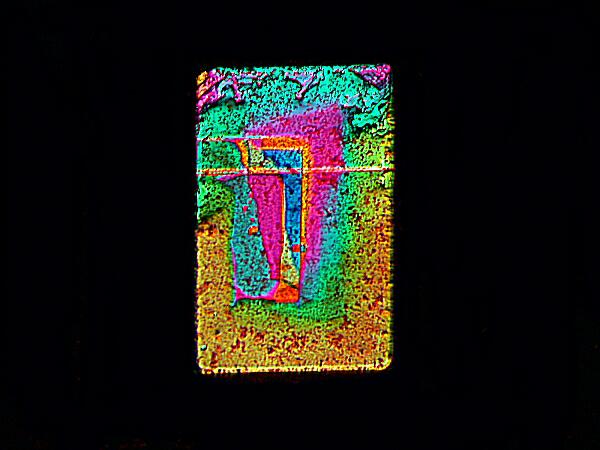
And finally this resplendently colored rectangle was achieved by mixing with another biological stain called Eosin Y. I hope you have enjoyed this mini-tour of the world of Magnesium chloride crystals.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK