
Robert Pavlis, Girard, Kansas USA
Sulphur is a rather abundant chemical element. It occurs on the Earth's crust in many places as the native element. Furthermore, there are a great many sulphur containing minerals that contain sulphur as sulphides and sulphates. There are large deposits of this native element in many places in the world. Often large masses of crystalline sulphur form around fumaroles in volcanoes.
The image below and to the left shows an area covered with sulphur inside the crater of Mount Misery (or Liamuiga) on the West Indian Island of St. Kitts. The image on the right shows a close up of some of the crystalline sulphur at this location. (A visit to this place involves a difficult and somewhat dangerous climb up to the rim of the a large crater, and then a very steep descent down to the crater floor.)

Sulphur has four stable isotopes, 32S (95.02%), 33S (0.75 %),34S (4.21%), and 36S (0.02%).
Sulphur exists in a remarkably large number of molecular forms, most of which are rings of various sizes. Many of these molecular forms are polymorphic, that is they have two or more different crystal structures from different packing arrangements.
The term allotrope is sometimes used to describe different forms of the same element. It is highly unfortunate that this term is generally used to describe ALL solid state variations of elemental substances including both structure differences and polymorphism when it should be used only for structural differences.
The most stable sulphur molecular structure at room temperature is a cyclic molecule, S8.
The image below was obtained by ab initio computation of the theoretical shape of the S8 molecule using the mpqc quantum chemistry program to compute the positions and the University of Illinois vmd program to produce the image. This image is indistinguishable from images obtained by physical measurement. (The ab initio techniques use only fundamental physical constants and quantum mechanics to determine structure and energy of molecules.)
Both mpqc and vmd are extraordinarily high quality programs. Both are available with complete source and without charge.
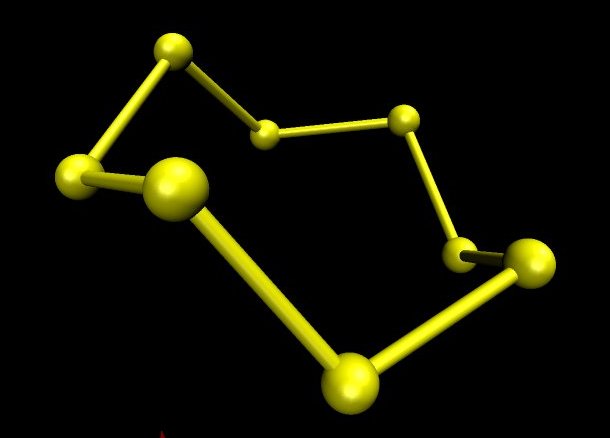
The S8 molecule is polymorphic with three well known crystal structures. There are many other cyclic molecule structures of sulphur known with other numbers of sulphur atoms in their rings.
The α (alpha) S8 crystal structure forms orthorhombic crystals. It is the most stable structure at temperatures below 95.3°. The density of these crystals is 2.07 g/cm3.
The β (beta) S8 crystal structure forms monoclinic crystals. It is the most stable structure at temperatures above 95.3° up to the melting point which is about 119.7°. The density of these crystals is 1.96 g/cm3.
The γ (gamma) S8 crystal structure is also monoclinic. It is unstable at all temperatures. It often forms when solutions containing elemental sulphur are cooled quickly. Because it is less stable than the other two structures it has a tendency to revert to the α or β form when in contact with sulphur solvents. The density of these crystals is 1.92 g/cm3.
It is difficult to obtain a true melting point of S8 because at temperatures in excess of 100° or so the S8 rings tend to begin to form other molecular structures that act as impurities and lower the melting point by several degrees. When β S8 is heated quickly it melts at about 119°.
Ordinary sulphur contains a small amount of S7. This structure is bright yellow. It has been produced in four different polymorphs. The image below is an ab initio one produced in the same way as the one for S8. When S8 is obtained so that it contains no S7 it is a very pale yellow, almost white.
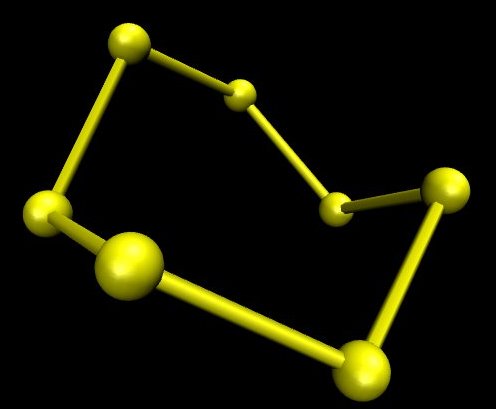
The energy differences between the different cyclic sulphur molecules are relatively slight, and the rate of their interconversion is relatively high. S8 is the primary structure at room temperature simply because it is a bit more stable, but because of the relatively small energy difference between it and other structures, pure S8 on standing soon becomes contaminated with other structures.
When sulphur is in the liquid state it quickly becomes complex mixture of molecular structural forms. The ring structures break open and recombine in many different ways. If held at high temperature for some time one forms what is commonly called "plastic" sulphur, which is a linear polymer of S atoms. This material has extraordinarily low solubility in solvents. It slowly reverts to S8 on standing at lower temperature.
When sulphur is heated to about 445°, it boils. The vapours, however, are S2 molecules, electronically analogous to oxygen. Sulphur vapour is dark brown.
Sulphur will burn in air producing sulphur dioxide. It can also be oxidised further to form sulphur trioxide. (Industrial sulphuric acid is made by hydration of sulphur trioxide.) The sulphur oxides are a common air pollutant.
Crystalline sulphur makes a truly wonderful subject for microscope observation. Large specimens from volcanoes and the like often make spectacular specimens for stereo microscopes.
However, one does not have to visit a volcano to observe sulphur crystals, and the visual impact of properly prepared specimens can be almost overwhelming!
The traditional way to prepare sulphur crystals for microscopic examination using polarising microscopes is to place a bit of sulphur in the centre of a microscope slide, add a drop of nitrobenzene and then a cover slip and carefully heat the preparation until the sulphur melts. As the sample is cooled crystals will form, more often than not, there will be crystals of all three of the S8 forms. The γ structure is particularly birefringent, and generally more of it forms initially than the other structures.
When materials have more than one polymorph, the less stable crystal structures generally convert to the most stable one. This process may occur in a fraction of a second, or over thousands of years, or any interval in between. They usually do this by one of two processes--either the more stable crystal structure begins to form spontaneously in the solid state, or, when the materials are in contact with solvents, the unstable crystal structure gradually dissolves and the material recrystallises on the surface of the stable form crystals that are nearby. Because unstable structures have lower crystal lattice energy, they are always more soluble than the more stable ones.
γ-sulphur is less stable than the either α-sulphur or β-sulphur at any temperature. It is therefore more soluble in solvents. Because of this when samples of sulphur crystals are suspended in solvents at room temperature the γ form slowly dissolves and the α form crystals grow. Eventually only α crystals remain.
The β-sulphur is also less stable than the α-sulphur at room temperature. The crystal formation β -> α seems to occur mostly without dissolution.
The crystals formed by crystallisation from solvent are quite large and are best observed with a polarising microscope using objectives from 2.5x to 10X.
There is one serious objection to this traditional method. Nitrobenzene is extremely toxic. I have prepared sulphur crystals for demonstrations in classes many times this way, always using a fume cupboard to contain the toxic vapours of nitrobenzene. There are a few people that use aniline instead of nitrobenzene, but it also is extremely toxic, nearly as toxic as nitrobenzene! A few people have used carbon disulphide. However, it is also extremely poisonous, it also has an extremely unpleasant odour, and it ignites spontaneously at only 95°!
I tried to think of a relatively common material that had low toxicity, did not smell bad, and that would dissolve molten sulphur. It seemed to me that benzyl alcohol should be a good substitute. It has unusually low toxicity, does not ignite very easily, and is a commonly available material. I put some on a microscope slide, and found that it worked quite well. Because sulphur seems to be somewhat less soluble in benzyl alcohol than nitrobenzene at room temperature the unstable polymorph crystals seem to remain longer because the material does not rapidly redissolve and then deposit on the more stable crystals.
The images below are all of sulphur crystals prepared in this manner. It is very important not to use too much sulphur. The best technique seems to be to place a small amount of powdered sulphur (about the size of a pin head) near the centre of a microscope slide. Add a drop of benzyl alcohol, cover with a cover slip and heat until the sulphur melts. Allow to cool. After it has cooled a bit watch the crystallisation proceed under polarising microscope. Molten sulphur is not miscible with benzyl alcohol, nor is it with nitrobenzene. Because of this if one use too much sulphur it will separate out as little spheres that will crystallise later into little balls of tiny crystals.
All of the images were obtained using a Canon digital SLR on a Nikon polarising microscope (S series) using a 2X Olympus projection ocular.
The first image shows all three forms of S8 sulphur using a 10X objective. The α- (rhombic) sulphur form tends to form compact blocks that when isolated often appear as elongated octahedra. The β-sulphur tends to form long branched dendritic crystal growth. The γ-structure tends to form wide highly birefringent "feathery" crystals that show brilliant colours under a polarising microscope. Surprisingly more of these unstable crystals initially form than the other two.
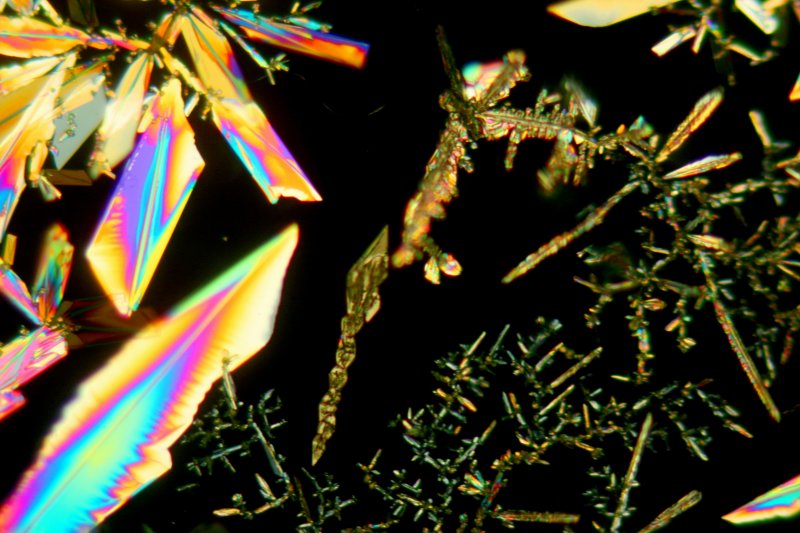
The image below shows a particularly interesting crystal, also using the 10X objective.
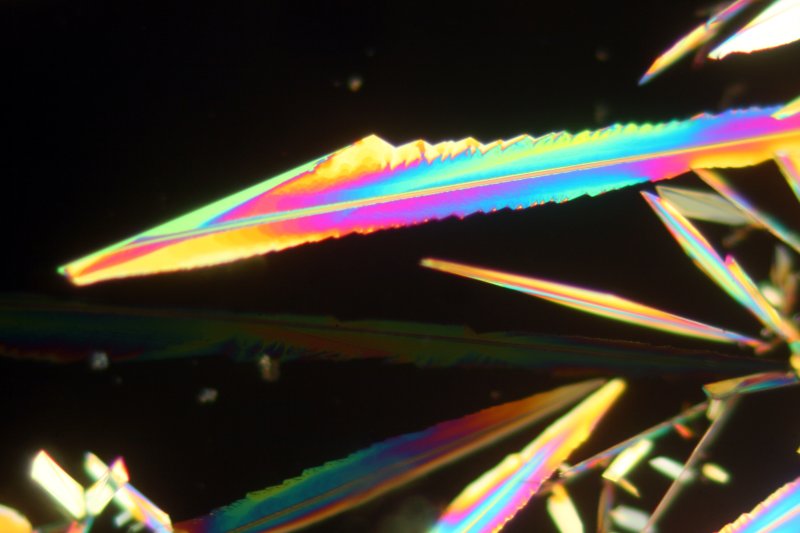
The next image shows a large group of crystals, again using the 10x objective.
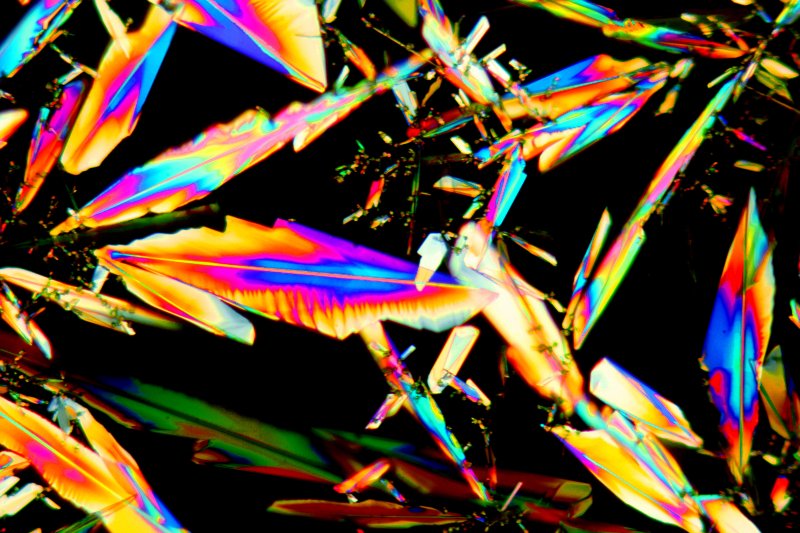
The following image shows a large field of view taken using a 2.5X objective. Notice the way that the polymorphs exist side by side. During crystallisation, once a crystal starts to form the S8 molecules in its vicinity tend to precipitate onto it.

The 10X objective image below is interesting. When the large crystal that runs from lower right to upper left was forming, it encountered a zone where the sulphur in solution had been depleted by another crystal, the greenish one trimmed with red that runs from lower left toward the right centre.
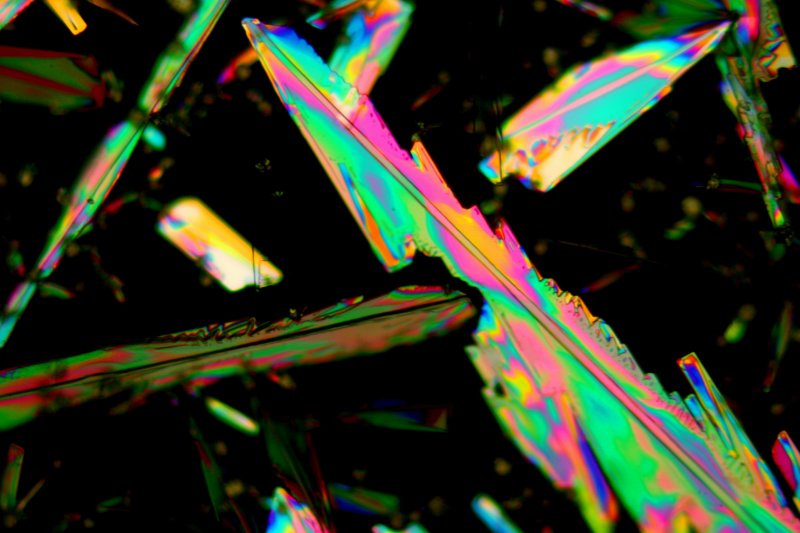
Because benzyl alcohol has a fairly low vapour pressure, it will remain on a slide preparation for a long time. I allowed the preparations that were used to make the above images to stand for thirty hours, and then I obtained more images.
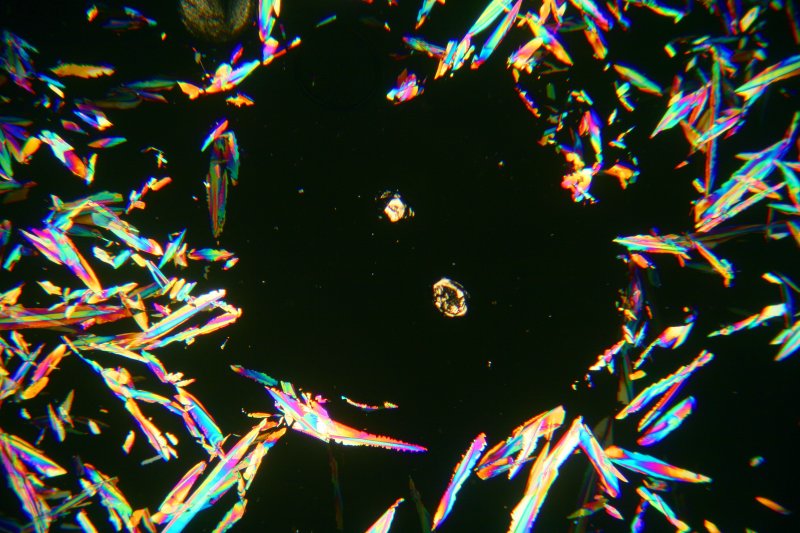
The image below is interesting. There is are two large α-sulphur crystals in the centre of the image. Thirty hours earlier the whole slide was rather uniformly covered with crystals of γ-sulphur. The dissolved sulphur near the α-sulphur crystals crystallised on their surfaces, and the nearby crystals of γ-sulphur began to dissolve, providing more sulphur in solution to crystallise onto the α-sulphur crystals. After 30 hours a nearly circular area of the slide has completely depleted of γ-sulphur, and the crystals of α-sulphur have become quite large. This image was obtained using a 2.5X objective.
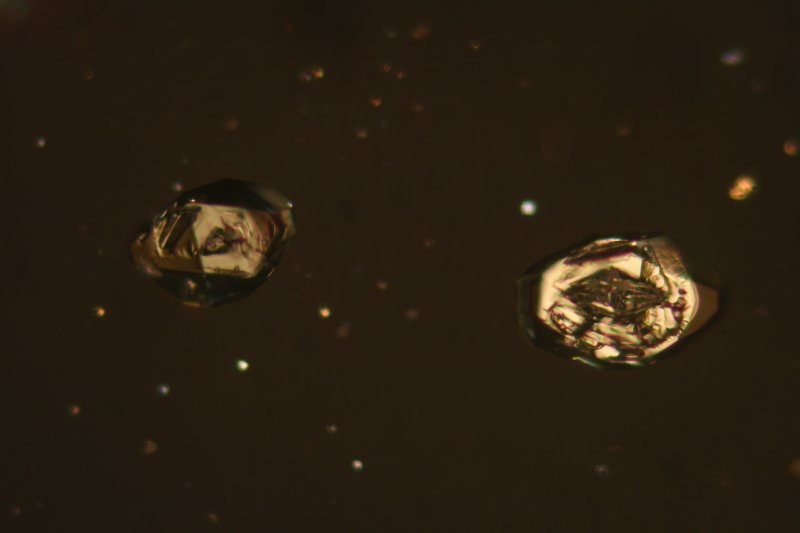
The image below was obtained of the α-sulphur crystals near the centre of the above images using a 25x objective.
The image below was also obtained after thirty hours. It was obtained also obtained using the 25x objective. It shows a mass of α-sulphur crystals that seem to have resulted from the transformation of β-sulphur crystals.
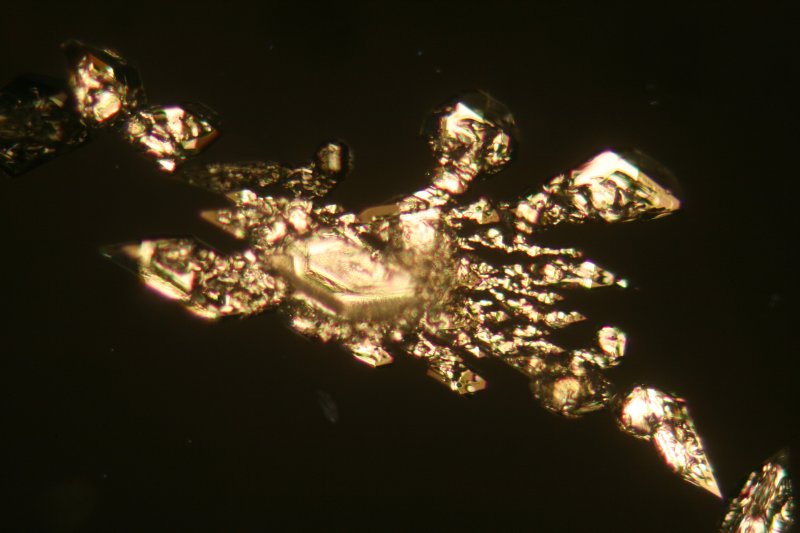
The image below was obtained using a 10x objective, again thirty hours after the slide was prepared. Note how these crystals of γ-sulphur have "eroded" from their slow dissolution as material was transferred to the more stable nearby α-crystals in their vicinity. In time no γ-sulphur will remain at all.

There is a lesson in the technique described above. It is very often possible to replace dangerous solvents with ones that are much less dangerous without any negative effects. Benzyl alcohol functioned at least as well for crystallising sulphur as the highly toxic nitrobenzene or aniline. Note that benzyl alcohol vapours will burn if one heat samples of it carelessly. One can, if careful, heat a slide with a drop of it under a cover slip over an open flame, though it is apt to ignite if heated too strongly.
WARNING: Do not attempt to do this with phenol. Phenol is very toxic and corrosive. (Benzyl alcohol is C6H5-CH2OH. Phenol is C6H5OH.)
Further WARNING: Do not attempt to do this with nitrobenzene or aniline except in proper laboratory facilities. Both of these compounds are highly toxic. Not only are their vapours exhibit both chronic and acute toxicity, they are also readily absorbed through the skin. Nitrobenzene also ignites rather easily. Our reference
All comments to the author Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK