|
|
A Gallery of Irreplaceable Photomicrographs
Melt specimens of thioacetamide,
testosterone by Brian Johnston (Canada) |
|
|
A Gallery of Irreplaceable Photomicrographs
Melt specimens of thioacetamide,
testosterone by Brian Johnston (Canada) |
There are many reasons why it may be difficult or impossible to reproduce a particular crystal photomicrograph. Some of my favourite images (shown here) were captured twenty-eight years ago using Kodachrome colour slide film and the equipment shown at the end of the article. Although I would very much like to retake them using my modern digital equipment, this is not possible. The reasons are varied.
Thioacetamide
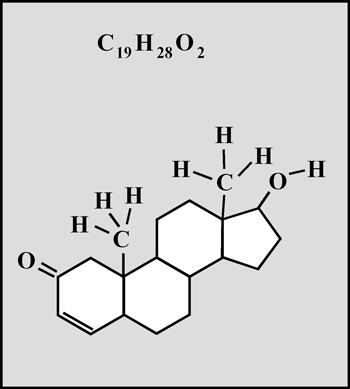
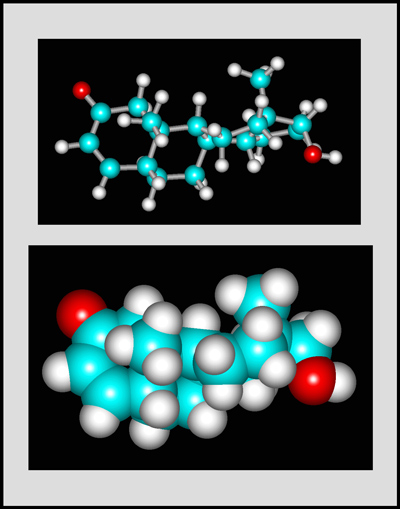
This organic compound is a white crystalline solid with a melting temperature of about 112° Celsius. (The molecular visualizations in the article were produced using "HyperChem".)
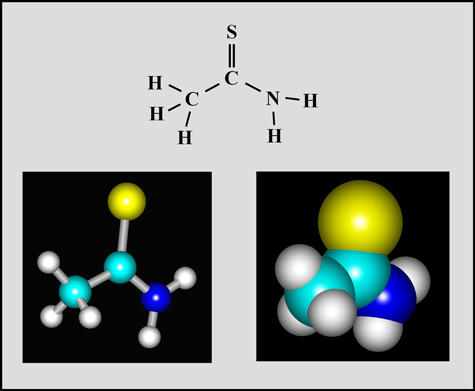
Thioacetamide was used for many years as a replacement for hydrogen sulphide in the qualitative analysis of inorganic compounds. During the 1970's in Ontario (Canada), large Secondary Schools had very comprehensive chemical inventories in their laboratory preparation rooms. No expense was spared to provide a wide selection of compounds, part to be used in the prescribed experiments in the chemistry curriculum, and part available "just in case". The modern concern with the dangers involved had not yet begun to surface. Thioacetamide was one of the chemicals in my inventory as a chemistry teacher in one of those schools. This would never be allowed today, since the compound is very poisonous if swallowed or inhaled. As well, thioacetamide is readily absorbed through the skin and may cause serious liver damage. It is now considered to be an "anticipated human carcinogen". (Whew! Am I glad that my lab had a large fume hood which I used when preparing melt specimens for observation!) During the last decade, most of the harmful compounds in school labs were removed and disposed of by professional hazardous chemical disposal firms. It is now difficult or impossible for an individual to obtain substances from chemical supply houses.
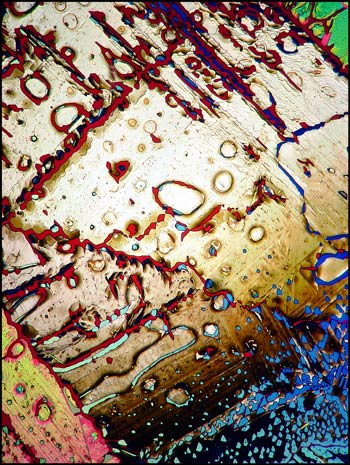
A typical crystal melt field can be seen in the first image in the article. Both lambda and lambda/4 plates were used in addition to crossed polarizers in order to produce the pastel colours in the image.
The photomicrograph on the left (below) was produced under similar conditions. The image on the right used only the lambda/4 plate in addition to crossed polarizers.

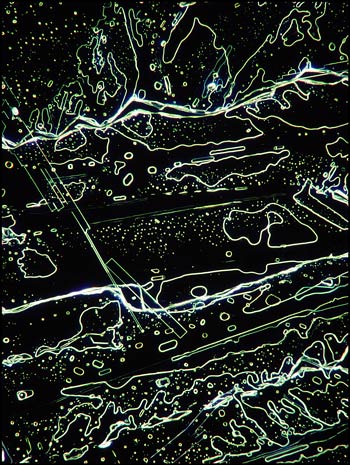
Although most thioacetamide crystal patterns appear rather random, (almost "modern art" in nature), careful examination reveals elongated perfectly rectangular formations sprinkled throughout the fields. The photomicrograph below on the left shows one of these in the middle left of the field. A similar field in the image on the right, viewed with dark-ground illumination, reveals a different perspective.

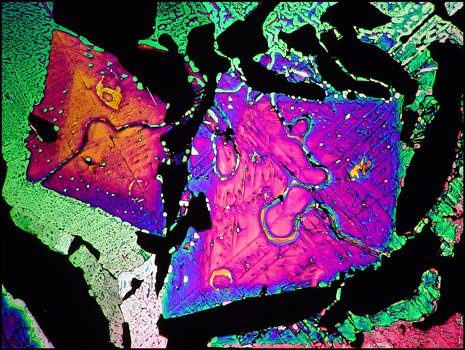
Thioacetamide is very volatile, and if the slide is reheated several times, sublimation results in empty gaps in the field which show as black between crossed polarizers. The image below shows one such field containing two colourful kite-shaped structures.
Testosterone
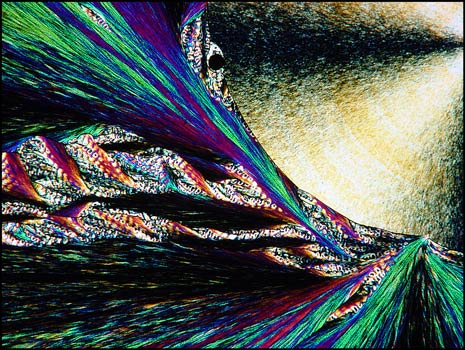
Testosterone is the steroid "male hormone" produced in the testes. Women also produce small amounts of this compound. It is a white crystalline solid with a melting temperature of about 155° Celsius.
Usually, when a melt specimen is cooled, crystals of the compound begin to form immediately. In some unusual compounds, crystallization may be delayed for a short time and the sample may not be completely solidified until several days have passed. When I attempted to produce testosterone melt specimens, they did not re-crystallize at all! After several days, I gave up the slides as a lost cause and stored them in my "reject box". Several weeks later, when disposing of the unsuccessful slides, I noticed very tiny crystal growths. Since they looked promising under the microscope, I put them away to continue to "grow". In fact, the slides took from six to eight months to crystallize completely! It was certainly worth the wait.
The first image in this section shows a typical field between crossed polarizers. The colourful "twisted ribbon" formations are characteristic of the compound.
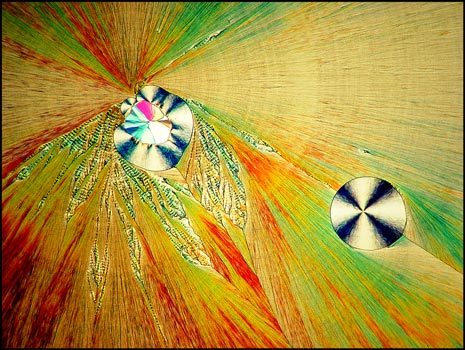
A much lower magnification shows the circular formations that often accompany the ribbons. A lambda/4 plate was used to produce the image below. The second image was produced at a higher magnification.

Other areas of the slides reveal curious fringed patterns, the colour depending on the thickness of the crystal layer in the chosen area.
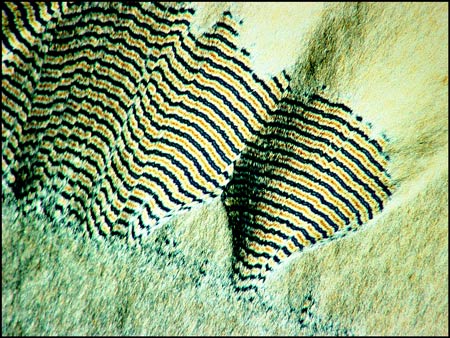

If the crystal layer is particularly thick, the fringes appear as black and white bands.
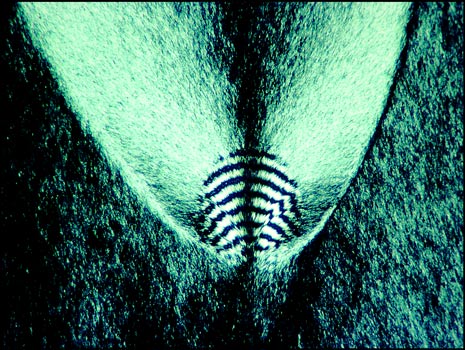
In order to preserve many specimens, I ringed the cover-glasses with "Permount". This usually worked wonderfully, and I still have many perfectly preserved melts after almost thirty years! Unfortunately, with testosterone melts, the Permount migrated over time into the crystal structure between cover-slip and slide and the mounts have been ruined. My only records are the colour slides taken originally. It is possible to obtain testosterone today (for instance from a University lab), but this compound is considered an eye and skin irritant which harms by ingestion or inhalation. It also may be a carcinogen.
Liquid crystal
In an earlier article in Micscape, I showed examples of this strange form of matter. As I was producing the melt specimens to photograph for the article, I kept looking for several unusual crystal formations that I had captured on slide film many years before. Unfortunately, all were illusive. This is strange, because I kept track of which numbered microscope slide was photographed to produce each colour slide, and I still have the actual samples. (Yes, I am perhaps a bit obsessive about organization and record keeping!) The following images are therefore the only ones in which I captured the interesting formations.
The tiny spherical growth shown below seems to be a very rare occurrence. It was photographed between crossed polarizers.
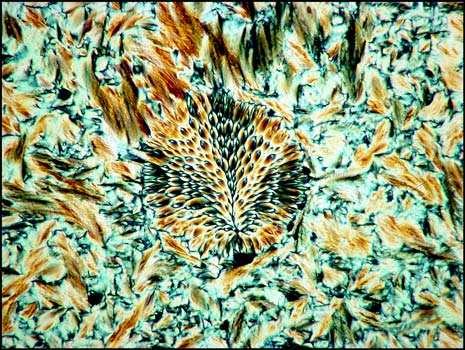
The rarest of all liquid crystal formations that I observed is shown on the right below. It was found in a field similar to the one shown in the image on the left. A lambda/4 plate was used in addition to crossed polarizers for both images.
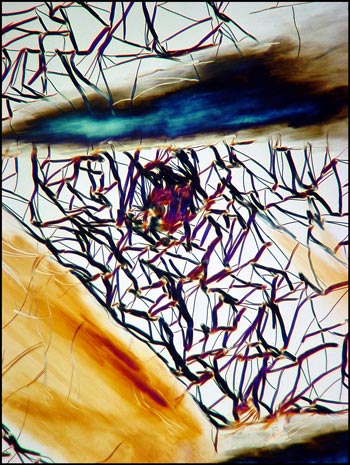
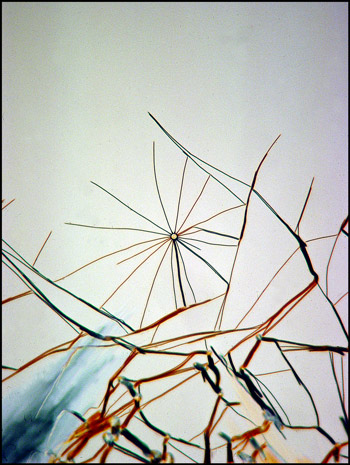
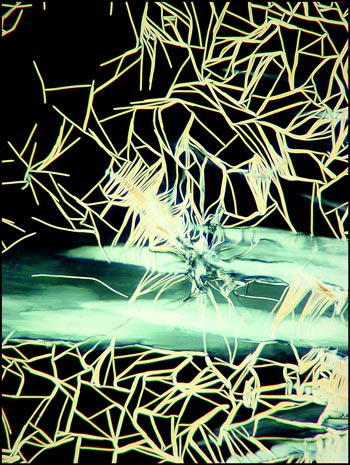
The image below left shows rod-like formations in a liquid crystal field. Strange comb-shaped growths are revealed in the image on the right.

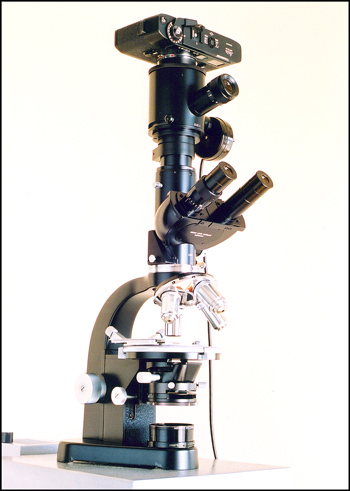
Photographic Equipment
The photomicrographs were taken using the Leitz SM-Pol microscope shown below. Once the field to be photographed has been chosen, the image is framed and focussed using the single eyepiece above the binocular head. The large diameter vertical tube beneath the camera contains a very effective dampening mechanism to reduce the vibration produced by the focal-plane shutter in the Leica M5 camera. In the right hand image, an exposure probe can be seen to the right with its wire connection to an exposure meter. (Since the M5 camera has an excellent internal exposure metering function, the external meter is used infrequently.)

All of the photomicrographs in the article were taken on Kodachrome 25 (!!!) colour slide film. (Remember that higher ASA Kodachrome was only a dream 30 years ago.) The images used in the article were produced by photographing the slides using a Sony DSC-F717 digital camera equipped with two Nikon 6T close-up lenses. A home-made optical bench assembly illuminated the slide.
All comments to the author Brian Johnston are welcomed.
Published in the May 2004 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line
monthly magazine of the Microscopy UK web
site at
Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net.