|
|
A Gallery of Urea Photomicrographs (using polarized light) |
|
|
A Gallery of Urea Photomicrographs (using polarized light) |
Urea is an important historical
compound. In 1773 Hilaire Rouelle discovered that human urine
contained this substance. At the time it was strongly believed
that the animate world bore no relation in chemical terms to the
inanimate world, (a theory called ‘vitalism’).
In simple terms, molecules found in living organisms were somehow
‘special’ and could not be prepared from the everyday compounds that
stocked a chemistry lab of the period. A little more than fifty
years after its discovery, in 1828, urea was the first organic
substance to be artificially synthesized in the lab. The chemist,
Friedrich Woehler, prepared urea from two ‘ordinary’ compounds,
potassium cyanate and ammonium sulphate, and thus showed that vitalism
was not a viable theory. The modern science of organic chemistry
was born out of the interest in molecules that play a part in the
processes that occur in living organisms.
Urea is used extensively in the
manufacture of urea-formaldehyde resins, which in turn are used to form
plastics. The compound is also involved in the production of
fertilizers which release nitrogen to plants to facilitate their growth.
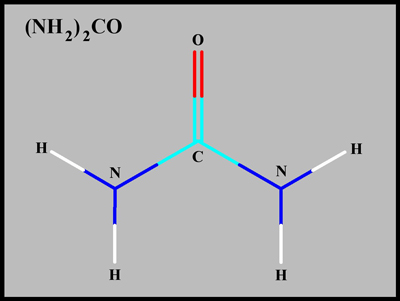
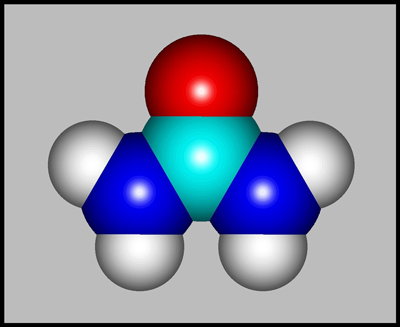
The structural formula and
molecular shape of urea are shown below. Compared with most
biological organic compounds, urea is relatively simple. (Both
illustrations were produced using HyperChem.)
Since the white crystals have a
relatively low melting temperature, (about 133 degrees Celsius), urea
makes an excellent melt specimen. Note however that the compound
is harmful if swallowed or inhaled, and causes irritation to the skin,
eyes, and respiratory tract.
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Crossed polars were used in all polarized
light images. Compensators, ( lambda and lambda/4 plates ), were
utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x
or 25x flat-field objective formed the original image and a 10x
Periplan eyepiece projected the image to the camera lens.
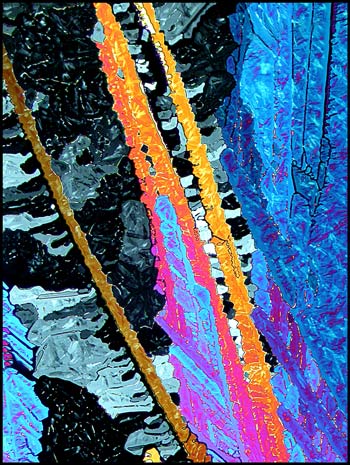
Provided that the thickness of the
crystal layer is suitable, urea can produce strikingly colourful
images. In the first image (above), lambda/4 and lambda compensator
plates were used to decrease the contrast. In the two images
below, only crossed polars were used. If the rotating stage of
the microscope was moved to a different angle, the dark areas would be
brighter and the bright areas darker. This particular orientation
was chosen to reveal the yellow-orange ‘arms’.


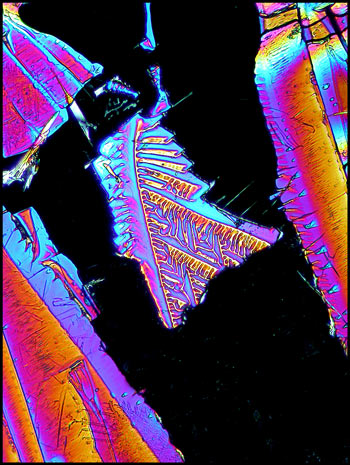
The following images show a higher
magnification view of the same area.

Sometimes the small details are more interesting than the overall
structure. The image to the right is a magnified view of the
small shape at the upper centre of the left image.

Consider the gray areas in the
image below.

Under higher magnification, and
with the aid of two lambda/4 compensators, roughly triangular detail
appears.
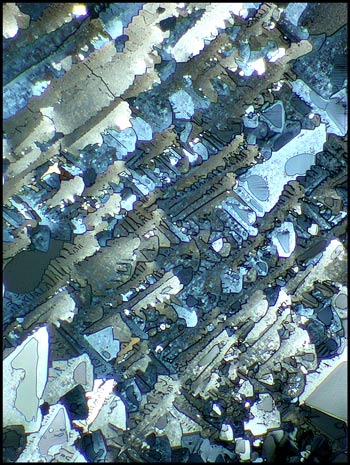

The first image in the article
shows what looks like blue-red quills on a feather. If these
quills are magnified, and both lambda and lambda/4 compensators are
used, one can see unusual details in the bands. (The left image
is my favourite urea photomicrograph. There’s no accounting for
taste!)


Here is a challenge. Can you
find the structure shown in the right image within the left image?


Small ‘blobs’ such as the one shown
below occurred occasionally in the darker areas. Compensators
reveal the complex structure in the area outside the ‘blob’.
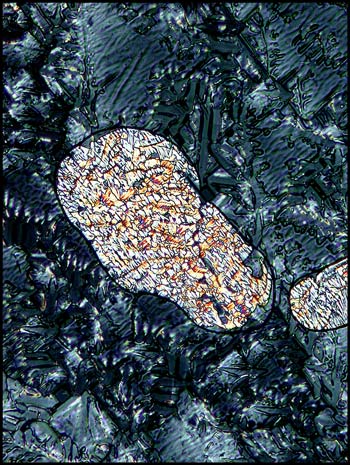

Under higher magnification, detail
is resolved in the outside area. The same magnification also
shows the subtle detail in a different ‘blob’.
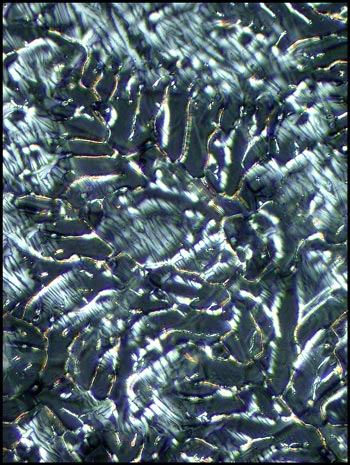
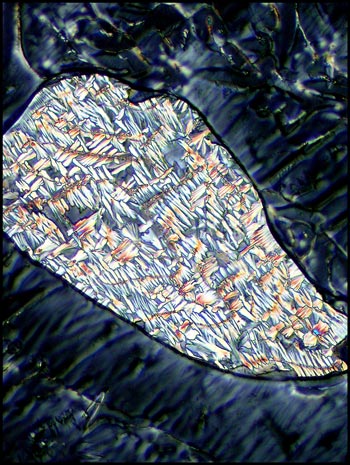
The two photomicrographs that
follow display the change caused by rotating one of the two lambda/4
compensators. Notice that each orientation highlights the detail
of different areas.
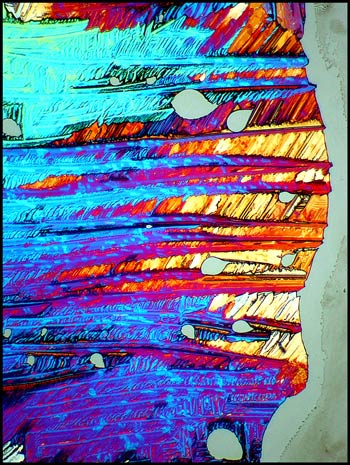

Consider the top right corner of
the left image below. Under much higher magnification, this
corner has a completely different appearance when dark-ground
illumination is used to reveal the detail of the crystal edges.

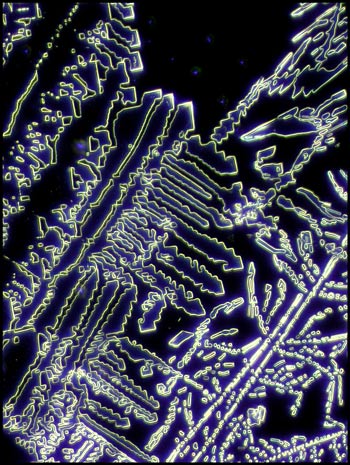
Urea it seems to me, is an ideal
substance to study under the polarizing microscope. It is not
extremely dangerous, and once the melt specimen has been prepared, the
sample can be re-melted many times to provide fascinating crystal
fields. Experiment by using a pencil eraser to push gently on the
centre of the coverglass while the compound is still molten. This
will provide a crystal thickness gradient which will result in more
interesting fields.
Equipment
The images in the article were photographed using a Nikon Coolpix 4500
camera attached to a Leitz SM-Pol polarizing microscope. Crossed polars
were used in all polarized light images. Compensators, ( lambda
and lambda/4 plates ), were utilized to alter the appearance in some
cases. A 2.5x, 6.3x, 16x or 25x flat-field objective formed the
original image and a 10x Periplan eyepiece projected the image to the
camera lens.
Published in the May
2005 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .