|
|
A Gallery of Nickel II Nitrate Photomicrographs (using
a variety of illumination techniques) |
|
|
A Gallery of Nickel II Nitrate Photomicrographs (using
a variety of illumination techniques) |
Nickel
II nitrate is commonly used in the electroplating industry as a supply
of nickel ions that are reduced to shiny nickel metal atoms on the
surface of “nickel-plated” utensils. Older chemists would have
used the archaic “nickelous nitrate” name to refer to the
compound. It is usually supplied as nickel II nitrate hexahydrate
crystals having a transparent green colour. (The hexahydrate term
means that six water molecules are associated with each nickel nitrate
molecule in the crystal lattice.)
The compound has a very low melting
temperature, 57 degrees Celsius, and therefore makes a good melt
specimen. Keep in mind however, that it is a very strong
oxidizer, and contact with other materials may cause fire or
explosion. It is harmful if swallowed or inhaled, and may cause
contact dermatitis. Nickel II nitrate is also thought to cause
cancer when a person is exposed over a long period.
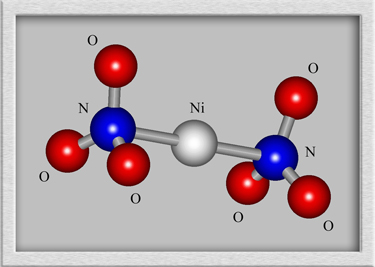
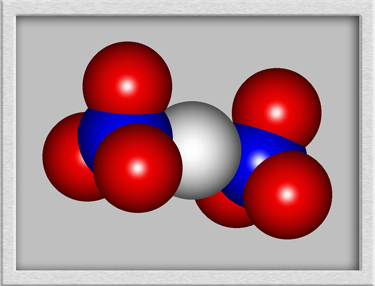
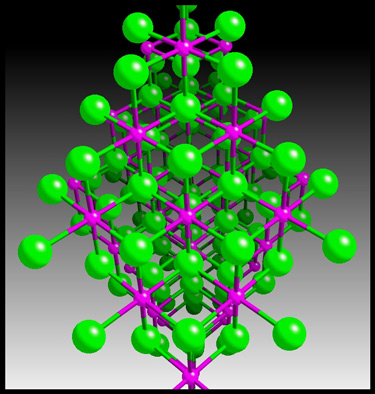
Although a structural formula and
molecular shape can be drawn for such a compound, the results are
misleading. Nickel II nitrate forms crystals, with the nickel
ions and nitrate ions arranged in a rigid three-dimensional
lattice. An example crystal lattice is shown below.
(“HyperChem” was
used to produce the first two images, and “CrystalMaker” generated the
third.)
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using several
illumination techniques: dark-ground, phase contrast and polarized
light. Crossed polars were used in all polarized light
images. Compensators, ( lambda and lambda/4 plates ), were
utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x
or 25x flat-field objective formed the original image and a 10x
Periplan eyepiece projected the image to the camera lens.
Nickel II nitrate melt specimens
tend to be difficult to photograph since the best sections tend to be
rather thick. The human eye, (and brain), do a wonderful job of
coping with a variety of focus planes. Cameras do not! The
auto-focus, (which I use constantly when taking photomicrographs),
sometimes chooses the wrong plane to focus on. Experience with a
particular camera usually provides a way to “trick” the mechanism to
focus on the desired plane by using a high contrast feature in the
plane as the focus point.
The two polarized light images
below show typical fields. Careful examination reveals tiny
“perfect” parallelogram-shaped crystals sprinkled over both
crystalscapes.


A higher magnification reveals that
the “perfect” crystals are actually quite “imperfect”. Corners
are sometimes missing, and bubbles often form within the growing
crystal.
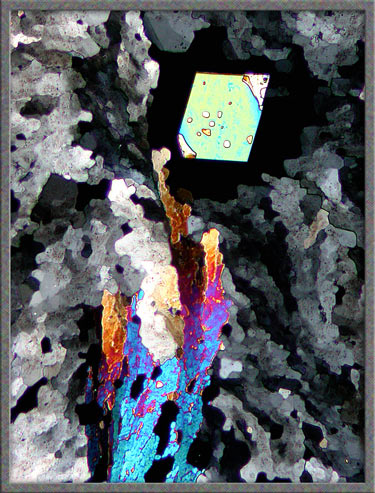



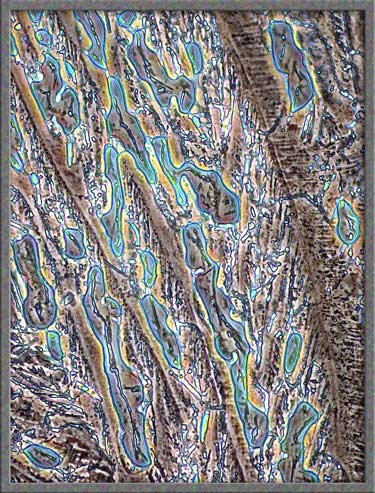
Other areas of a slide may contain
intricately mottled sections like the one below. (Both images are
of exactly the same field.) Compensators were used to alter the
colours.
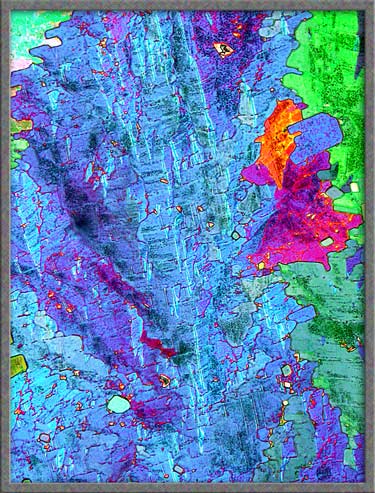

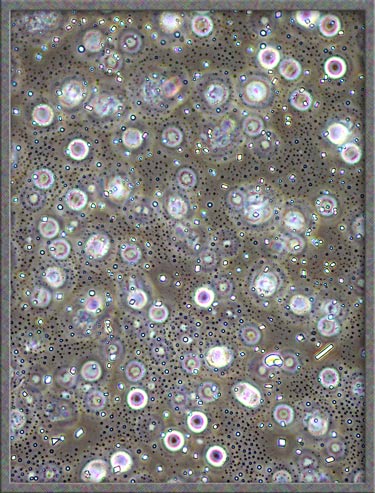
Most areas contain circular, or
elongated “specks”. The right image shows a magnified picture of
an area in the left image. Again, compensators were used to
produce a different colouration.
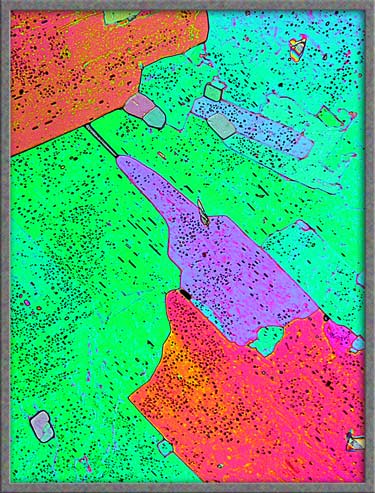

The transmitted light image on the
left is rather boring when compared to a polarized light image of the
identical field shown on the right.


Three images of similar areas are
shown below.



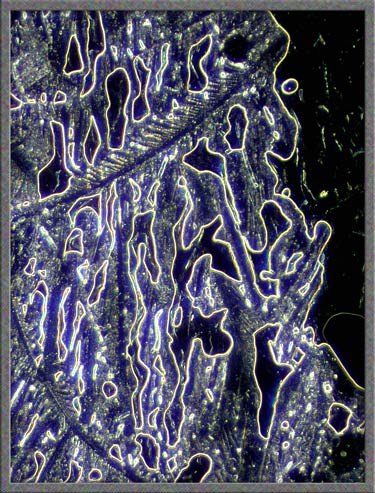
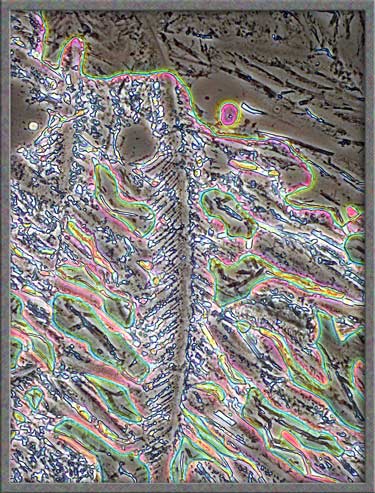
Dark-ground illumination can be
used to show a different perspective of the thicker sections of the
melt. A polarized light image, (using compensators), of the same
field is shown on the right.

Dark-ground illumination provides a
clear view of the “perfect” parallelogram-shaped crystals in a
particular field.
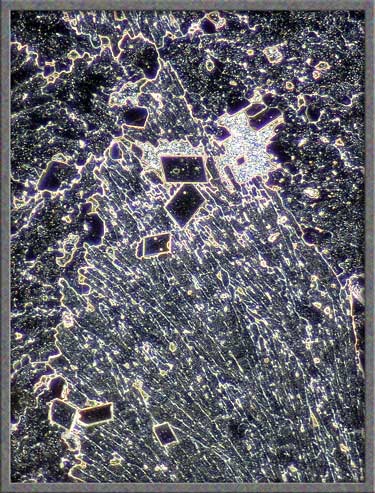

The
thicker areas of the melt also provide unusual views with phase
contrast illumination.


Nickel II nitrate hexahydrate, in
my very humble opinion, is not one of the “stars” of the crystal
universe. It requires more effort, and produces less photogenic
images than most other compounds that I have photomicrographed.
All comments to the author Brian Johnston are welcomed.
Published in the May
2006 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1996 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .