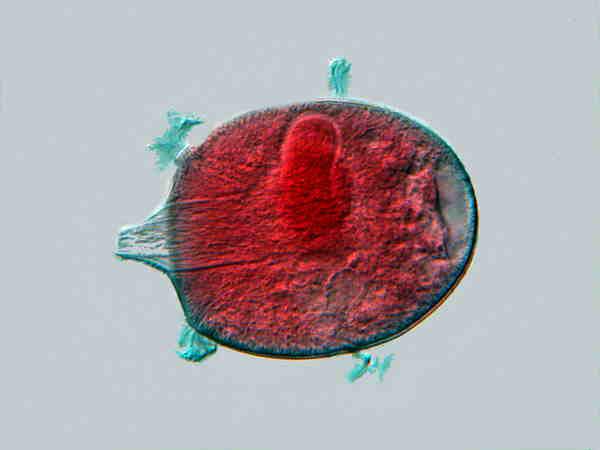
Didinium
|
Encystment and Excystment Excitement (Try Saying That Fast 10 Times) by Richard L. Howey, Wyoming, USA |
Any of you who have spent much time looking under logs and rocks have probably encountered (in addition to politicians) an interesting little organism called a sowbug or pill bug. The latter name is quite descriptive since, when disturbed, they frequently roll up into a little ball and play dead. They have armor plates and look rather like a land-locked chiton with legs. To my mind, they are rather charming little creatures which I’m not going to talk about here except as an example of a rather clever survival strategy.
In discussing encystment and excystment as a survival strategy, I am going to limit myself to protozoa. These remarkable phenomena are “the stuff that dreams are made of.” Imagine if we had the ability to go into a state of suspended animation when our environment got too polluted or our psychological stress became overwhelming or our physical condition had seriously deteriorated. Now, further imagine that you could initiate this process of going into a suspended state without being dependent upon any kind of technology–no cryogenics, no liquid nitrogen, no phenomenal medical fees. However, the best is still to come; while you’re in this blissful Nirvana, if you’re old, decrepit and falling apart like me, you can undergo a series of processes of genetic repair and when conditions are right, re-emerge as a vigorous and productive organism–no surgeons, no chemical injections, no life-support systems, all of this is built right into your biochemistry. Well, O.K., not yours, but rather that of these primitive, useless, little microscopic creatures. A bit humbling, isn’t it? But maybe someday we’ll unravel their secrets and be able to apply them to ourselves, if we don’t eliminate ourselves as a species first through our greed, hatred, stupidity, technological recklessness, and poisoning of the environment. However, on my good days, I’m an optimist and believe that the creative human spirit can transcend even moronic politicians–eventually, but it had better be sooner than later.
At one time, it was rather blithely assumed that virtually all free-living protozoa with the exception of some marine forms and some freshwater ones that lived in very large bodies of water, were capable of encystment. However, now, aquatic microbiologists are much more cautious and tend to approach the issue from the reverse direction; that is, don’t assume a protozoan encysts or excysts unless you’ve observed one of these processes in a specific organism. It used to be standard to regard encystment as a survival strategy of Paramecium , but now, it’s not so certain, since it has never been well-documented. Didinium encystment is well-documented and you can order them in this state, place them in a rich, active Paramecium culture and get lots of Didinia popping out to prey on their favorite food. I’ve tried this 3 or 4 times with no satisfaction of my bloodlust to see Didinia feasting on Paramecia. I don’t seem to be able to get the conditions right.

Didinium
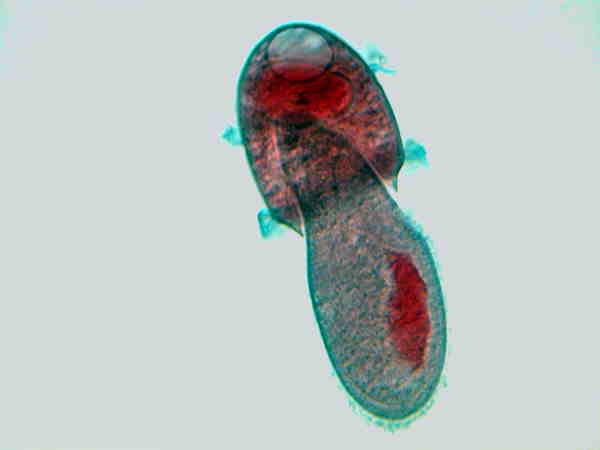
Didinium eating Paramecium
These images do show the predation, so we know that’s a fact. Or did I fake the images and create them by means of computer graphics software? Did I bribe the Didinium ? As President Bush would say: Trust me! Well, if you don’t believe me, you can order slides from several biological supply houses. I have, however, personally observed Bursaria truncatella and Colpoda cucullus encysting (Bursaria ) and excysting (Colpoda ). Images of either process are rather scarce, but with the advent of digital photomicrography and its increasing accessibility to amateurs, I suspect that in the near future many more such images of an increasing variety of protozoa will become available.
Some cysts, especially in their early stages, like those of Bursaria are quite readily recognized, but this is the exception and certainly not the rule. To observe encystment, the best way of proceeding is to use a “pure” culture, that is, one that contains only a single species of protozoan and food organisms, such as, bacteria or algae. When the culture is flourishing, radically alter its conditions by reducing the food source and light. For example, if you have been providing bacteria as a food source by adding rice or wheat grains, stop adding any additional grains and remove the old ones from the culture and then place the dish in a dark place where it will receive no light. You will need to check your cultures frequently and very carefully examine the bottom of the dishes with the highest power of your stereo-dissecting microscope. Alternatively, you can use a micro-pipet to suck up some of the debris from the dish, deposit it on a slide, place a cover glass on the drop, and then examine it with a compound microscope to see if you can find any indication of encystment. One reason for maintaining a “pure” or monoculture is that there are a number of other micro-organisms other than protozoa which also form cysts or produce eggs which often look like cysts. Rotifers are a prime example.
Some of the larger ciliates and amoebae are extremely difficult to culture without prey organisms being included in the culture. Didinium will only thrive on Paramecia or other mid-sized ciliates; Bursaria can gracefully slide a Paramecium down its gullet; Coleps also seem to have a preference for Paramecia and once a single Coleps , which is significantly smaller than a Paramecium , makes its strike, it seems that a biochemical signal is released and other Coleps quickly appear , feeding on their prey like jackals. Stentor coeruleous and Blepharisma are voracious feeders. I have seen a S. coeruleus with 4 sizeable loricate rotifers which it had ingested. One of my former colleagues, who was a cell biologist, told me he had observed the same phenomenon in an even more extreme form, wherein the Stentor had ingested so many rotifers that it finally burst open having no way to get rid of the loricas. In these multi-organism cultures, trying to track for encystment of a given organism is much more difficult. It makes one long for a nice Spirostomum culture which is packed full of fecund organisms, a few wheat grains and lots of bacteria–no other protists. Such cultures are easy to start and easy to maintain. Spirostomum is also a marvelous organism to observe. I describe them as micro-whales and I very much enjoy their slow, elegant progress as they move through the water. With enormous numbers of these large protists in a culture, one would expect that cysts would be relatively easy to find when conditions become unfavorable. In over 50 years of observing protozoa, I cannot say that I have ever seen a Spirostomum cyst. So what’s the problem? Spirostomum , like virtually every organism, has its little idiosyncratic tricks. Remember that S. ambiguum and S. minus are giants in the world of protozoa and can attain sizes of 1000 to 3000 microns! They have a remarkable device for startling predators; when disturbed they contract to about half their full size and with such incredible rapidity that this feat has, perhaps hyperbolically, been described as the fastest known response in the biological world. In any case, it’s fast enough to elicit astonishment from virtually anyone who observes it for the first time. Spirostomum isn’t fussy about bacterial food and likes a rather rich organic environment. In fact, in a pond out in cattle pastures, I have seen cow patties with white masses 5 or 6 inches in diameter which were conglomerations of enormous numbers of Spirostomum . However, when you get them in culture and try to study them, their hyper-contractility makes your efforts considerably more difficult. My few efforts to alter their conditions to see if I could induce encystment have met with consistent failure.
Sometimes I have taken random samples off of the bottom of a variety of different sorts of cultures and then scanned them at medium to high power with a compound microscope looking for cysts. Usually if I find some, they turn out to be rotifer cysts or eggs.
One protist that is reasonably cooperative regarding encystment is Colpoda cucullus. It is of moderate size–about 100 microns and fairly common not only in ponds, but in soil samples. Of course, you want to select rich, moist soils to place in your culture dishes. Colpoda are capable of a neat trick when they undergo encystment–they reproduce by dividing. [For a nice discussion of this process along with drawings from direct observation, see Rose Marie Arbur’s article.] This remarkable strategy should make us pause and reflect on the whole concept of encystment. It may turn out that there are several different phenomena that get lumped together under the term ‘encystment.’
I am familiar with three different processes that involve a sort of “hibernation.” 1) There are those cases which are clearly encystment and are a survival strategy to deal with adverse conditions. 2) There are those cases, such as Colpoda , where, in a relatively, protected state, reproduction can take place. 3) There are “resting states” wherein a protozoan contracts and becomes inactive. This is especially common after having a first-class meal at a five star restaurant. Lacrymaria olor often demonstrates this behavior after feeding and it is clearly a short-term state like sleep in humans. If you stir things up on the bottom of a seemingly dead Lacrymaria culture, you may quickly find some Lacrymaria swimming around rather lethargically, grumpily, and usually erratically; in fact, sometimes in this state, they will swim backwards. On a number of occasions when active and “resting” Lacrymaria have disappeared from cultures, I have tried reviving the cultures by adding fresh Giese salt solution and a boiled wheat grain, but with no positive results. I’m strongly inclined to believe that Lacrymaria do indeed form cysts, but I haven’t yet been able to demonstrate it, so my belief remains in the realm of protozoan theology and not scientific fact.
These processes are remarkably complex and researchers continue to learn more and more about the biochemical and biophysical mechanisms involved. As humans we can imagine enormous benefit to ourselves from all three processes. I’ve already mentioned some of the advantages we could gain by being able to encyst and excyst. We’ll turn briefly to the topic of excystment in a moment.
As for the encysted reproductive strategy of Colpoda , many human females would, I think, regard being able to reproduce while in a hibernative state as a distinct advantage, particularly when, after excystment, all of the “offspring” are able to feed independently and can go off immediately and start their own lives. No teenage Colpoda to care for!
Lacrymaria’s prolonged “resting stage” after a heavy meal has already been incorporated into a number of human cultures and is especially noticeable after holiday feasts, such as, Thanksgiving and Christmas.
Finally a few remarks on excystment without which, of course, there would be no point in encystment. In the “genuine” cases of encystment, leaving aside the issue of different types, some remarkable conditions must be met. Let’s consider the most extreme kind of case and I’ll use my own environment as an example. The area where I live is 7,200 feet high, on a dry prairie with temperature and moisture extremes possible during both winter and summer. Strong winds are frequent with gusts up to 60 or even 75 miles per hour. In the 40 years that we have lived here, we have seen temperature extremes from -39 degree Fahrenheit to 95 degrees Fahrenheit. The last 6 years, we have had drought conditions, although last “summer” on June 4th, we had 13 inches of wet snow as heavy as concrete and for 2 months, it was enough moisture to keep things lush and green. Why–you ask–would anyone want to live in such an inhospitable place? Well, Steamboat Springs in Colorado, about 125 miles southwest of us, has already gotten 400 inches of snow this year–yes, 400 inches! And the winter isn’t over yet, but it makes Laramie seem quite comfortable. Dry, clear air, crisp in the mornings, over 300 days of sunshine per year, 12,000 foot mountains with spring alpine meadows resplendent with wildflowers, the scent of pine, wild orchids (I’m not joking), eagles, pelicans, blue herons, moose, elk, coyotes, brown bears, porcupines, red-tail hawks, and lovely lakes filled with an extraordinary range of micro fauna and flora.
So, what does all of this have to do with excystment? Let’s consider a sort of idealized generic ciliate. In this climate around Laramie, it has to be able to survive, among other things, dehydration. Interestingly, some protozoans when they encyst form not just one protective “wall”, but as many as 4 or 5. Inside the organism has “dedifferentiated” itself; in other words, it has essentially become a blob of cytoplasm with genetic material. In addition to dehydration, our ideal cyst must be able to withstand freezing, heat, and higher intensities of ultraviolet radiation than at sea level. Furthermore, it is not ingesting any food to fuel its metabolic processes so, in terms of energy consumption, it is reducing it to near zero. This is an amazing set of accomplishments! A wide range of organisms other than protists have developed a series of such strategies ranging from rotifers and tardigrades to mammals that have a built-in “anti-freeze”. There have been some exaggerated reports regarding longevity of organisms in these states and from what is known now the record seems to be something over 30 years for certain nematodes. Some scientists have made some rather wild predictions about the possibility of significantly extending the human life span in just a few decades suggesting that humans could double or even triple the current life span. However, for the moment, say we could extend our lives by 125 years through going dormant. Imagine going into excystment in 1900 and then re-emerging in 2025. In 1900, no cars to speak of, no jet planes, no thermonuclear weapons, no computers, no TVs, no RVs, no stereo, no iPods, no cell phones and we’ve still got 19 years before we get to 2025. I suspect that on being revived, a massive cardiac infarction might be a rather common response.
However, to my mind, the most astonishing part is yet to come. Think of our ideal cyst battered, frozen, overheated, and blown by the wind until finally it lands in a lovely, woodland pond in mid-spring–a pond full of nutrients, a comfortable temperature, and plentiful food. At this point, our ideal cyst “knows” that conditions are good and it starts reconstructing itself. This blob “creates” itself anew. The triggers for this process are undoubtedly highly sophisticated biochemical and biophysical processes. There is, of course, another crucial factor–water. The outer cyst wall must be such that at an ultramicroscopic level moisture can gradually penetrate, carrying with it subtle biochemical messages regarding the surrounding conditions. If it had turned out to be just a little temporary puddle, all of this significant effort might have very well been for naught, but we’ve already got our cyst in a nice pond. So, what happens next? Well, if there are multiple cyst walls, then the appropriate signals have to get through those as well until finally something wonderful and extraordinarily impressive starts to happen. If this cyst were of a common species of Paramecium , for example, consider the range of morphological features that would need to be reconstituted. [Important Note: There is no definitive evidence that Paramecium encysts, in fact, the dominant current view is that it is primarily transported on the feet of waterfowl from lake to lake or pond to pond. I am using it as an example here because its morphology is better know to amateur microscopists than virtually any other ciliate. Furthermore, it is a fairly good analog to Bursaria which is known to encyst.]
So, if we take our hypothetical Paramecium (frankly Paramecia are so ubiquitous that I’m secretly inclined to believe that they do encyst), it must shape its macronucleus, and at least a couple of micronuclei, a Golgi apparatus, mitochondria, ribosomes, a cytostome, cilia, a cytopyge, trichocysts, a contractile vacuole and the canals leading to it, and so on. Such a critter deserves a Nobel prize for bio-engineering!
When I searched the internet for ciliate encystment, I found relatively little information on different species of ciliates where encystment is documented. There are a fair number of technical papers investigating the biochemistry and ultrastructure of the process. It is well known that a number of different genera of amoebae encyst, including some very nasty pathogenic forms, and that many sporozoa go through complex life cycles that include encystment. Documentation regarding encystment in flagellates is rather scarce. For example, I recall only coming across passing references to Volvox cysts. Are there Euglenoid cysts? Apparently some euglenoid cysts have been found and a number of papers have been done on dinoflagellate cysts, in part, because of the economic consequence of toxic “red tides” resulting from “blooms” of dinoflagellates. In general, most of the work on cysts has been done on parasitic or pathogenic protists.
I didn’t think I would be able to show you any images of cysts and then I remembered that several years ago, I had some excellent cultures going which produced some good examples of cysts and that I had isolated a few and placed them in vials of distilled water. It has been estimated that the average period of viability for ciliate cysts is between 4 and 8 years, however, such estimates have little significance given the limited amount of research on this problem. The images of the cysts which I have all fall in that time frame and we’ll consider the viability issue when we look at specific images. I have some decent images of Dileptus cysts along with some other smaller cysts which I suspect were some small ciliates that Dileptus was feeding on.
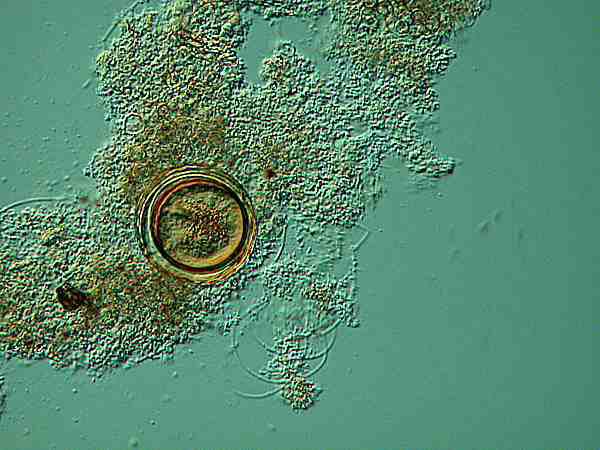
Even at this magnification, there is a suggestion of multiple membranes and no suggestion of specialized organelles within the cytoplasm.
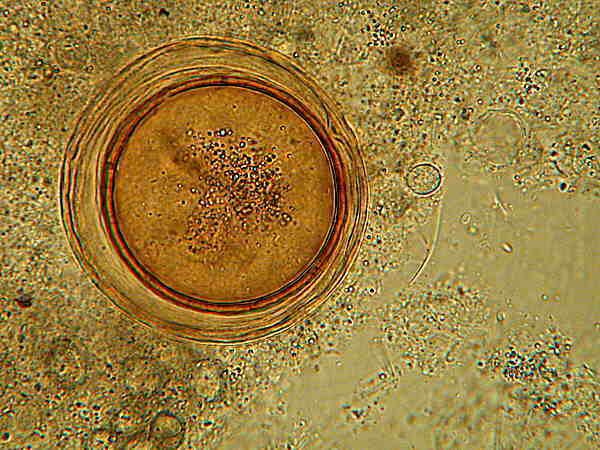
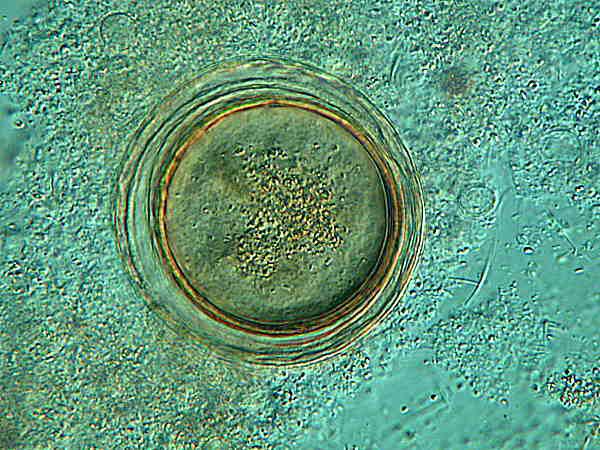
Here at higher magnification, it is evident that there are 3 or perhaps even 4 membranes. The upper image is brightfield and here especially at bottom left, you can see the smaller cysts which I mentioned. The lower image is Nomarski Differential Interference Contrast and because of the “optical section” associated with this technique, the small cysts are not as clearly visible, but the layering in the cysts “walls” of the larger Dileptus cysts is somewhat sharper. It is interesting to note that the innermost “wall” is thicker and appears to be double. In addition, the image, especially at the top of the cyst, suggests 3 other membranes or “walls”. As for the cytoplasm, there are definitely inclusions, but this is insufficient to determine whether or not the cyst is still viable. It is 4 years old and thus fits the time frame.
After observing this cyst carefully, I decided to stain it with Schneider’s Aceto-Carmine which I selected because of its vivid staining power and its rapid penetration provided by the 45% Glacial Acetic Acid and therefore should be handled with proper precautions. I thought that it might reveal some additional detail in both the cyst wall and the cytoplasm.
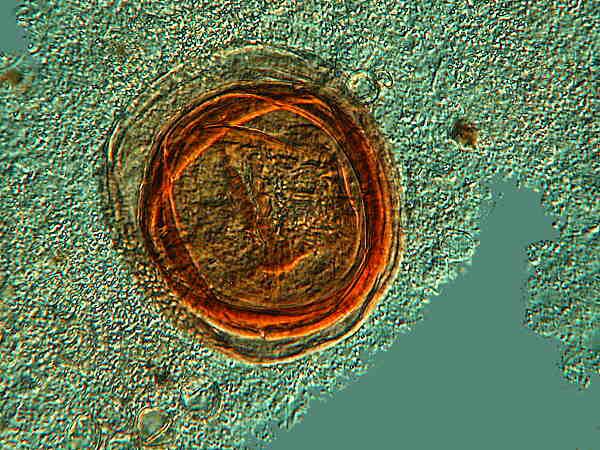
As you can see, there is indeed an increase in contrast, but otherwise, especially with regard to the cytoplasm, the staining simply raises additional questions. At this particular focal plane, it appears that there may be a fold in one or more of the cyst membranes which, if true, thus further obscures any detail in the cytoplasm
I also have a vial of cysts labeled Blepharisma , that wonderfully odd critter which has a unique pink pigment called, logically enough, blepharismin. This pigment is photo-active and if the organisms are exposed to too much intense light, the pigment becomes toxic and even lethal. These are also those strange protists which under certain conditions form “cannibal giants” which begin snacking on their own kind. Most of the cysts which I have are like round-bottomed vases and some of them are attached to bits of debris at the oral end.
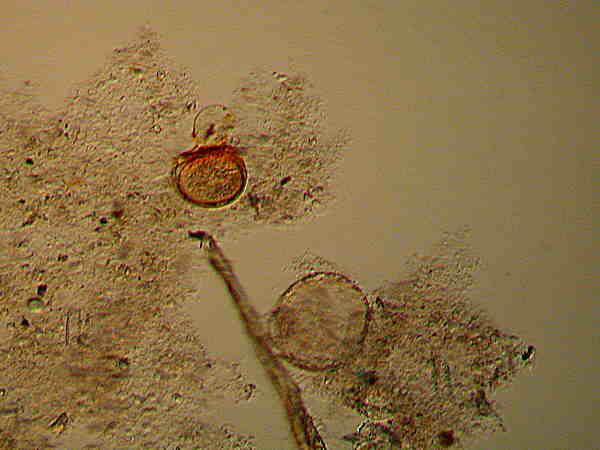
Notice that near the center there is a flattened cyst which retains some pigment. Also on the larger cyst, there appears to be a few grains of pigment on the outer wall. All of these specimens have been stored in distilled water and some may still be viable and none of these have been stained.
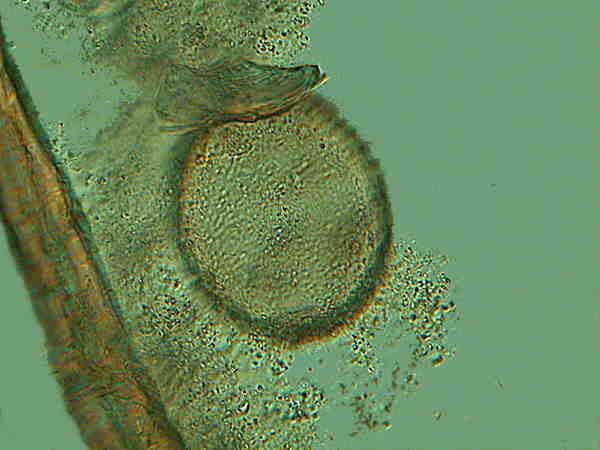
Above is one of the vase-shaped cysts still sporting its attaching debris. At the oral surface, there is a distinct trace of pigmentation.
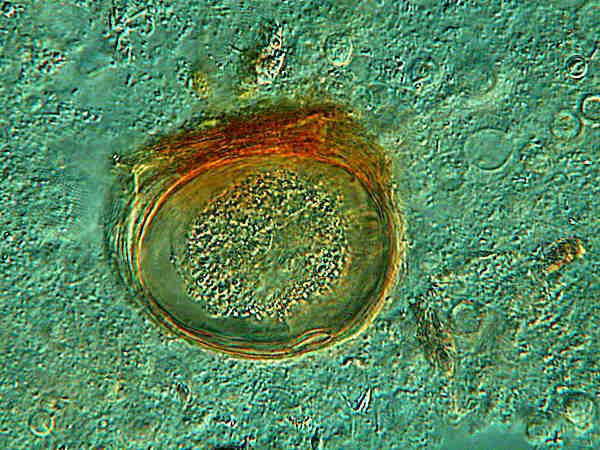
Above is a closeup of the flattened cyst. Again this is an image taken with Nomarski. Here it is clear that the pigmentation is extensive in the cysts’ membranes with hints of it in the cytoplasm as well, In this case, as in the Dileptus cyst, very little can be deduced from the inclusions in the cytoplasm. In one image which I took, there is vague structure that might possibly be a cluster of nuclear material, but such an interpretation is so tenuous that I have not included the image. Nonetheless, such inclusions do show that the cysts are not completely atrophied and void, so there is a possibility that they are still viable.
A third vial was unhelpfully labeled “unknown cysts”.
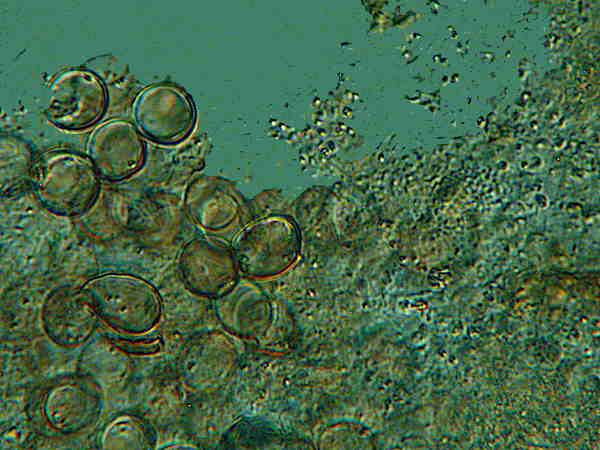
Clearly these are not Dileptus or Blepharisma cysts because 1) they are too small and 2) they are discernibly structurally different and, at this point, you know that it’s not true that if you’ve seen one cyst you’ve seen them all. Nonetheless, when you take samples from the bottom of cultures that have been around for a while and carefully examine them at 200 magnifications or more, you will discover all kinds of things you can’t identify and some of them will be cysts. However, no one, so far as I know, has ever been so foolhardy as to attempt a “catalog” of cysts.
Finally, I have a vial of cysts labeled “cysts from small fishbowl. Frontonia ?” These cysts are also several years old and, at this point, I have no idea whether they are Frontonia cysts or not. All I can do is hope to get a good culture of Frontonia this summer, try to induce encystment, and then compare them.
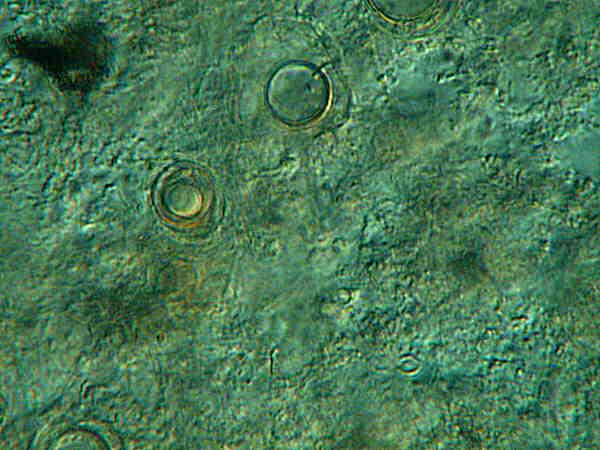
Note here that there are 2 cysts apparently at different stages.
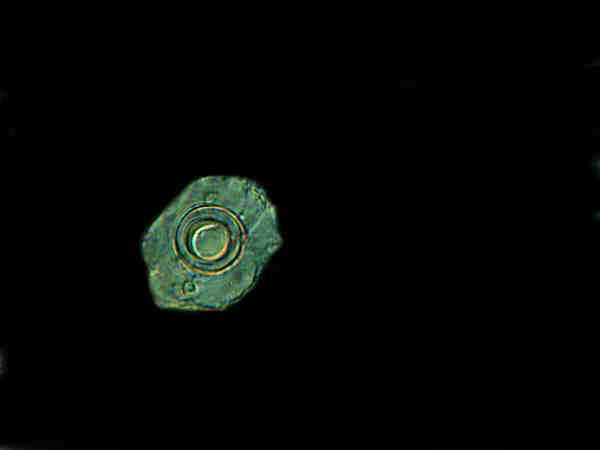
In this image of yet a third cyst, there seems to be a combination of elements of the 2 cysts in the image above this one. (I have provided a black background to emphasize the details.) The outer membrane is like that in the upper cyst of the first image and the center is more like the double-walled lower cyst.
Cysts serve a variety of functions and some are merely “resting stages”, some are reproductive, some are protective only in an aqueous environment, and others can survive heat, dessication, and freezing. These latter are most likely the rarest and most complex. Here is an area in which amateurs can make some important and fascinating contributions by carrying out a series of carefully controlled experiments. If you do try it and get some interesting result, please share them with the readers of Micscape .
All comments to the author Richard Howey are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK