Alkoxybenzoic acids
Robert Pavlis
Girard, Kansas USA
For pure samples of all compounds there are one or more types of attractive intermolecular forces that can act on molecules that are close to one another. When these attractive forces are strong relative to the thermal motion of the molecules the molecules tend to stack into a three dimensional crystal lattice. There are 230 different space groups that give rise to six crystal types: cubic, tetragonal, hexagonal, orthorhombic, monoclinic, and triclinic.
When the temperature of any compound is raised sufficiently the thermal motion eventually disrupts the crystal lattice and a liquid results. Generally at still higher temperature molecules escape from one another's attractive forces and the material enters the vapour state. Thus we have the three classical states of matter: solids, liquids, and gases.
When some crystalline solids are heated they do not lose their three dimensional order all at one time. Instead they form a "meso phase" or "liquid crystal" that still has one or two dimensional order. A material that still possesses one dimensional order is referred to as a "nematic" material. The name comes from a Greek word for thread. When nematic materials are examined between crossed polarisers under a microscope the samples tend to appear to have "threads" running through them.
Smectic materials (from a Greek word for soap) possess two dimensional order. There are several different ways to arrange molecules so that they possess two dimensional order. The two most common are smectic A and smectic C. Smectic A samples have the molecules arranged in parallel rows and smectic C samples have the parallel rows twisted at an angle. Some compounds can form several different types of smectic stable, each stable over a different temperature range. Bis(p-heptyloxybenzylidene) p-Phenylenediamine, (CH3(CH2)6OC6H4CH=NC6H4N=CHC6H4O(CH2)6CH3) for example, forms five different smectic phases!
When chiral molecules (those that are not superimposable on their mirror images) exhibit liquid crystalline properties the rows or planes of molecules tend to be twisted relative to their neighbours. The most common molecules of this sort are esters from steroidal alcohols, especially cholesterol. Sometimes nematic liquid crystals of this sort are referred to as "cholesteric". However, similar behaviour occurs with smectic materials.
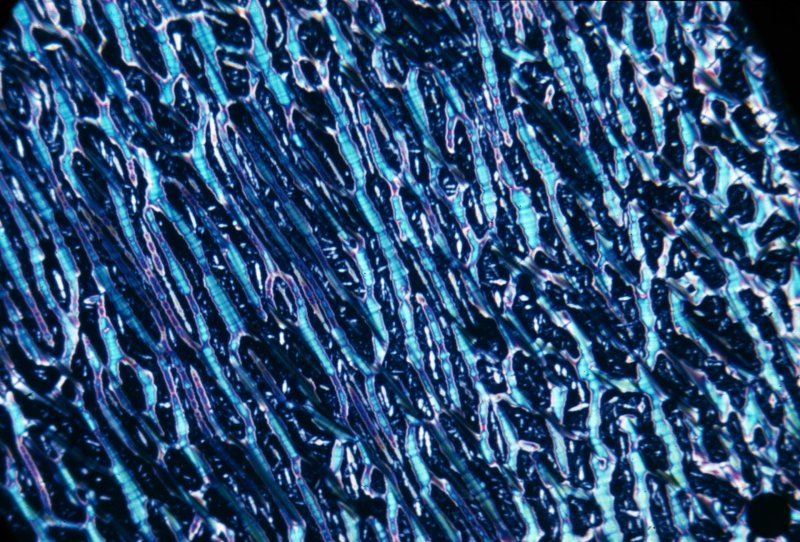
Because liquid crystals are optically anisotropic, they are birefringent like non-cubic crystals. Extraordinary patterns appear when they are observed under a microscope using polarised light.
The image below shows an image of cholesterol oleate. This image was taken many years ago.

The majority of liquid crystalline forming molecules that are not steroid derivatives have a long straight chain hydrocarbon on each end. These are generally attached to benzene rings, and some sort of polar group sits in the middle. The chemical and thermal stability of these materials differs widely, largely depending on what the polar group is. Imines are rather unstable and subject to hydrolysis. There are a great many that form liquid crystals. Azoxy derivatives are also often liquid crystals. They are not especially stable either. Two classes of compounds tend to form liquid crystals are are extremely stable: p-alkyloxybenzoic acid and esters of these acids formed with alcohols that permit building long rod like molecules that have straight chain saturated hydrocarbon chains on their ends. The esters tend to exhibit nematic phases not much above room temperature, but generally do not exhibit smectic phases at all. p-alkyloxybenzoic acids with from three to six carbon atoms on the alkyl side chain exhibit nematic phases. The ones from 7 up to about 13 or 14 on heating first form a smectic phase that then forms a nematic phase on further heating.
The phase transition temperatures for some of these compounds are shown below:
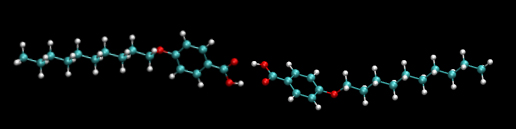
A peculiar characteristic of almost all organic acids is that they form dimers. The dimer of p-decyloxybenzoic acid is shown above.
The various p-alkoxybenzoic acids are truly fantastic substances to examine under a polarising microscope! If one has a microscope with an heated stage one can place a small sample of one of these materials on a slide and place a cover slip over it and then start heating it while watching it closely all the time it is being heated.
Actually, it is not necessary to have an heated stage microscope to do this. One can place a few crystals near the centre of a microscope slide, cover them with a cover slip and then heat the slide carefully with an alcohol or Bunsen burner until it completely melts. One can quickly place the slide on the microscope stage and watch the material as it cools down.
p-decyloxybenzoic acid is a good example of a material that exhibits both a nematic and a smectic phase. On heating it melts to smectic C at 97 degrees. At 122 degrees the material undergoes a phase change to nematic. Finally at 142 degrees the material melts to an ordinary isotropic liquid. If one then turns off the heater and allows the material to cool it will go through the same phase changes in reverse. However, when it gets to about 97 degrees it will often crystallise to an unstable polymorph that quickly reverts to the more stable form, this change is also very dramatic.
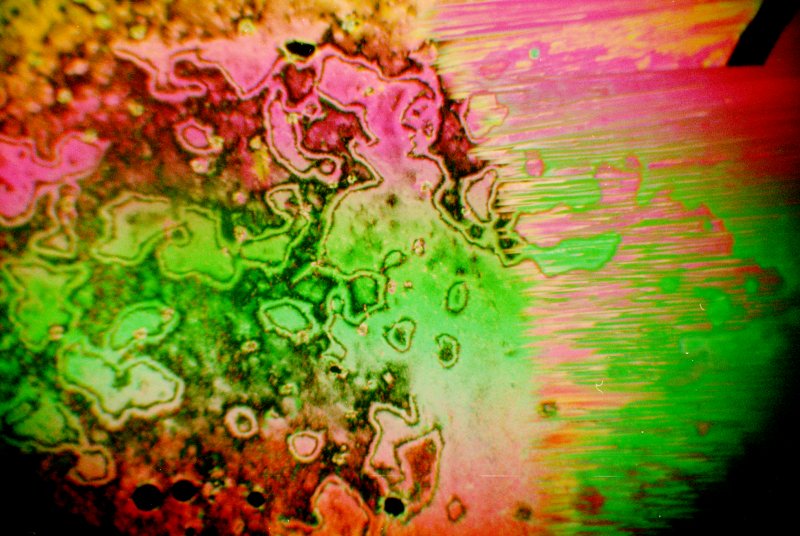
There is often an intense display of colours when the material is undergoing the phase transformation to nematic to isotropic or the reverse. The image below shows hexyloxybenzoic acid as the nematic phase is beginning to form from the isotropic phase.
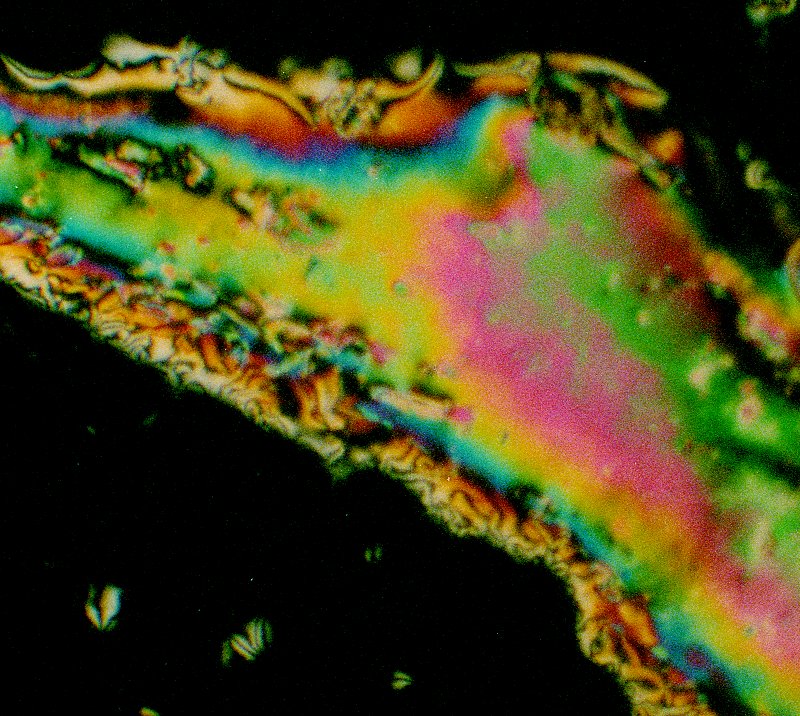
The image below shows hexyloxybenzoic acid between crossed polarisers in its nematic state. The colours are dramatic, and change somewhat with temperature.
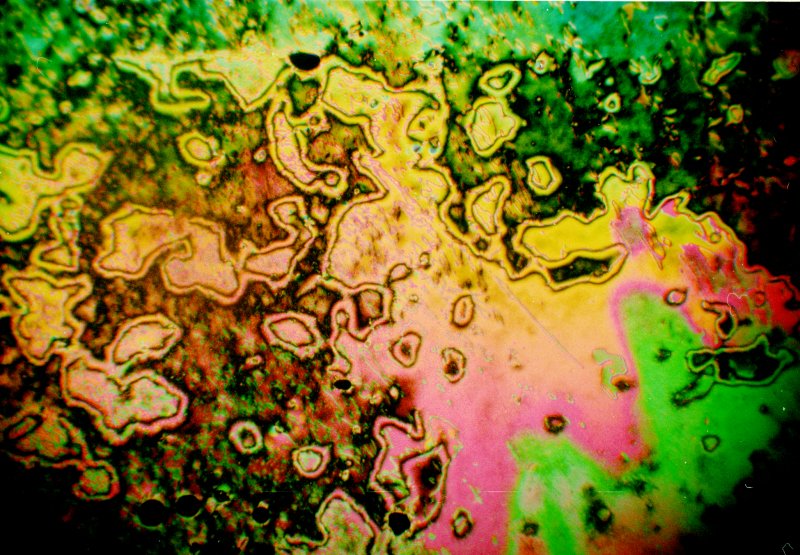
The image below shows decyloxybenzoic acid undergoing the nematic to smectic phase transformation as the sample is cooled. The phase transformation is proceeding from left to right. The nematic phase on the right side of the image shows the thread texture that gives this phase its name.
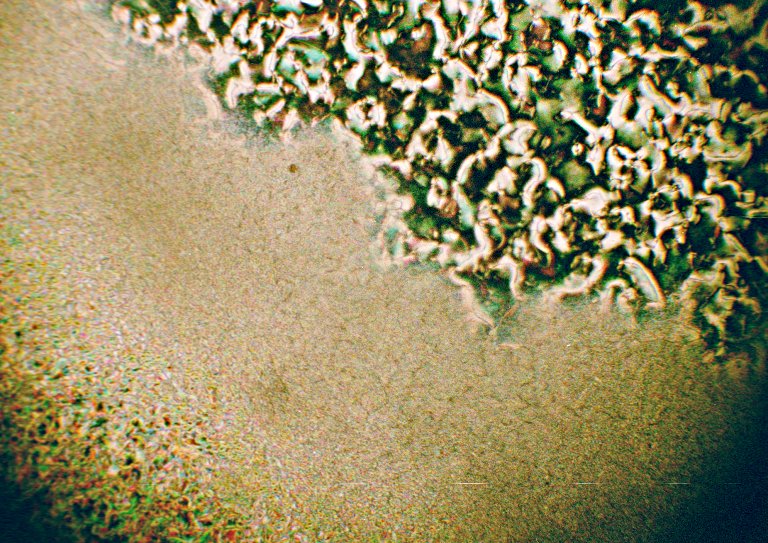
The image below shows hexyloxybenzoic acid during cooling while it is going from its nematic phase to crystal.
All of these compounds are interesting to observe. The ones with smaller chains do not exhibit a smectic phase at all, but the nematic phases seem to exhibit stronger polarisation colours.
After one starts observing these materials, one tends to want to observe them again and again. Because alkyloxybenzoic acids are so stable one can heat and cool samples over and over again and watch the fantastic show again and again.
Several p-alkyloxybenzoic acids are available from chemical suppliers. (For chemists: These compounds are very easy to synthesise. One can dissolve 21 millimoles of KOH in methanol and then add 10 millimoles of p-hydroxybenzoic acid. Do NOT use NaOH, it does not dissolve in methanol well enough for this synthesis. The mixture can be stirred and then 11 millimoles of 1-bromodecane (or other 1-bromoalkane) can be added to the mixture. The reaction is quite slow at room temperature, it requires several days to go approach completion. It may be better to seal the mixture into a glass tube, place the glass tube inside a short section of heavy steel pipe capped on each end, and then place the pipe in an 100 degree oven over night. The steel pipe can be removed from the oven, cooled completely to room temperature, and then the glass tube can be broken and the the mixture can be diluted with water and acidified with dilute hydrochloric acid. The alkyoxybenzoic acids will generally precipitate. They must be recrystallised from heptane or similar solvent to produce material of sufficient purity for viewing these phase transitions.)
All of the images were obtained using a strain free 4X objective on a polarising microscope. It is best to use low powers for observing preparations of these compounds.
All comments to the author Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK