
|
Mud From Miletus by Richard L. Howey, Wyoming, USA |
Over the years, I have been fortunate enough to have a number of special friends who have indulged my eccentric interests in microscopy and natural history. Several of them have brought me sand samples from a variety of exotic locations around the world, others have given me mineral and plant samples from their collections, yet others have given me samples of foraminifera and other micro-fossils, and a few have generously presented me with microscope slides both from their own collections and slides which they themselves made. Such thoughtfulness and kindness shows what a strong bond natural history can be.
The most recent gift was given to me by a wonderful person named Birgit Burke whom I have known for a number of years through the university. While taking an art class which involved a trip to Turkey, she visited the Temple of Zeus at Miletus. The floor of the temple was covered with several inches of water and mud; she saw little beasties swimming around and immediately thought of me and using a small tin which she used to clean her brushes, she scooped up a sample and after it had dried, deposited it in a plastic bag and brought it all the way back to Wyoming.


When I went over to her office to get the sample, she said: “I wouldn’t have done this for anyone else. If there’s nothing in it, you can add it to the soil around one of your houseplants.” The sample was a rather undistinguished gray, dried mud color and didn’t look very promising. I dumped half of the sample into a Syracuse watch glass and began examining it with my dissecting microscope. Gray, brown, graying-brown, and then a tiny bit of green which was a dried bit of moss or some kind of aquatic plant.

Then I had a glimpse of white–a small fragment of quartz and then a short white cylinder which looked like a piece of very thin plant stem encrusted with alkaline salts. And then, off to the side, a tiny valve of a shell perhaps. I took a fine needle and moved the dirt to get a better look and then lost it, but a minute later as a I poked and probed, this came into view.
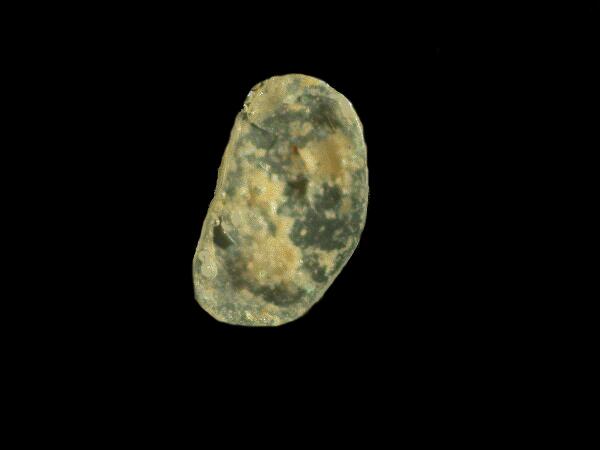
I recognized it at once–an ostracod shell. Within minutes, I had found a couple of dozen such shells, some still having both valves, some just one, and some that were just broken fragments and one of them was green!
Ostracods occur in both marine and freshwater environments and they have been around a very long time. I am going to devote the rest of this essay to taking a closer look at ostracods, but I want to mention one other item which I found in this mud sample–a reproductive part of the freshwater plant called Chara which also grows in quite alkaline waters. It too has ancient fossil representatives. Here is one that I found in the mud from Miletus.

Ostracods have not received as much attention as many other aquatic invertebrates for several reasons, one of the primary ones being that they are rather difficult to study and species identification often depends upon micro-dissection and the rather tricky business of counting bristles on specific appendages. Furthermore, they have no real economic impact except perhaps for a few species that act as intermediate hosts for parasites that infect a few species of fish and waterfowl. This means that one is very unlikely to get a grant to do ostracod research. Nonetheless they are intriguing little creatures and deserve a bit more press than they have gotten.
Ostracods are also known as seed shrimp and often do look like tiny seeds to the naked eye. Adult species in the U.S. range in size from about 1 mm. to 3 mm. Some fossil species and a few extant marine species can achieve a length of up to 25 mm. As they swim through the water, they appear–at least to the older low-tech video game cognoscenti–like Pac-Man critters.
They are busy little creatures and should certainly be pleasing to anyone driven by a Protestant work ethic. There is a fairly wide range of color and patterning. Some species are a single, solid color, but others, on close examination, will show subtle variations of pigmentation. The two valves of the shell are impregnated with calcium carbonate and other calcium salts and so many species have a chalk-like coloration. These are sometimes mottled with a variety of other colors. Here in the high altitude ponds and lakes, the colors which I encounter most are gray, brown, and green. Since, in instances like this, our means of designating colors are rather primitive , we have only vague linguistic approximations. It is in such cases as this that one longs for the great 19th Century illustrators and their lovely and intricate hand-colored drawings. Other colors of valves that have been reported are a dark purple-black, yellow, and red and, of course, combinations. One could spend a long time just studying the valves of these organisms which, by the way, consists of two plates with a thin layer of tissue in the center which produces the material for the construction of the valves.
If you wish to study the valves in detail, that turns out to be relatively straightforward in some respects. Ostracods are fairly common amongst certain types of algae, sphagnum moss, and other aquatic plants and are often found feeding just above the surface of detritus in quiet pools. Skimming with a dip net will usually provide some specimens. I often use a pint jar which I overload with vegetation. When I get back in the lab, there are two major ways of processing such material.
Method 1: Take strands of algae or moss and place them in large culture dishes and shake them vigorously and then place the plants in a separate container. A drawback of this method is that if you have a lot of material, you can use up a lot of culture dishes and require considerable space. Then you are faced with the rather tedious job of extracting the ostracods by means of a pipet. I take a disposable Pasteur pipet and carefully wrap the tip with a sturdy cloth and break off the end so that I have an opening which will accommodate the size of the ostracods. One quickly learns how to be fast with such a pipet. Preservation is essential for any detailed study, so after you have collected them and examined the living organisms to observe general external structures and behavior, you can pop them into some 50% alcohol as a killing fluid and then transfer them to 80% plus alcohol to store them.
Method 2: This is the method I prefer, because I am congenitally lazy. I bring my pint jars stuffed with aquatic vegetation back to my lab, take off the lids and let them ripen (and ripen they do–after a few days one needs a gas mask). There are several advantages to this method. First of all, you can often find a few ostracods swimming around on the sides of the jar or near the surface and having now perfected your fast pipet technique, you can capture a number of them. After 3 or 4 days, a thick brownish scum starts to form and so now, you begin to get added value. Last week, I collected some samples and 2 of them were just like this. Today I looked at a couple surface scum samples and in the first, I found Paramecia (of course!–wonderfully reliable critters), two species of hypotrichs, another small ciliate, and lots of other extremely wee beasties that require at least 1000x to see any detail. However, the important thing is that if you sample the bottom before it gets too utterly vile, you can often find numbers of dead ostracods, sometimes with the valves open revealing the critters, this being a consequence of asphyxia. Or if you take the samples outside and use a pair of tongs or long forceps to remove the vegetation you then can add some tap water and allow it to sit until it semi-purifies itself. Then after a couple of such rinses, letting the sediment re-settle, you can carefully pour off the water and use a wid-bore pipet to transfer some of the sediment to a Petri dish or watch glass containing clean water. You should now have numbers of ostracod shells. Some of them can be dried and stored and others should be preserved in strong alcohol.
I took a single valve and a complete shell from the sample; they were encrusted in mud and I put them in some 3% hydrogen peroxide–Houston, we have liftoff--immediately there were lots of bubbles indicating that there is a considerable amount of organic material in this marvelous Miletian mud. What surprised me is that the valve and shell began to break apart into fragments. Now, admittedly, they have been subjected to some fairly harsh treatment through drying and then rehydration and were as a consequence quite fragile even before being subjected to the hydrogen peroxide. Some additional checks of the literature revealed that the valve consists of an internal and an external “plate” separated by a thin layer of tissue which “produces” the valves. As so often occurs in nature, we here again have a very complex interaction of organic and mineral materials. So, in addition to the organic material in the mud, we have the peroxide acting on the tissue separating the “plates” of the valve.
The vales of some species have distinct sculpting or other features which can be an aid in identifying genera and, in some cases species. “How difficult can it be to classify them?” you might ask. After all, there are only a few hundred known species in the U.S. And there is the key difficulty: that word “known”. There are very few areas that have been systematically surveyed for ostracods and I am certain that in the high alpine lakes, ponds, and streams here, there are a number of unidentified species. Recent estimates are that there are over 35,000 species, about 15,000 of which still exist, although many of these are marine. Nonetheless, it is evident that there are several thousand freshwater species worldwide and it is likely that at least that many more that haven’t yet been identified. Lake Baikal alone would probably contribute dozens of species.
I strongly suspect that the color of ostracods is helpful in identification only in living specimens. I now have in one pond sample, a number of ostracods with a distinct forest-green color. However, in taking bottom samples to examine valves, I find none with pigmentation.


In many instances, I am inclined to believe that the pigmentation is largely a result of the habitat, specifically the types of food available and the minerals present in the water and debris. For example, the green ostracods tend to occur in environments rich in algae and the reddish-brown ones in areas where there are large quantities of iron minerals.
The internal anatomy of ostracods is complex and rather too complicated to describe in a short essay, so I suggest that you consult a good textbook on invertebrate zoology–some of you remember what books are; they’re those things with pages that in ancient times (pre-1990), people used before widespread access to the Internet was available. You will almost certainly find some helpful drawings showing you the relationships of various structures.
WARNING!
This next section is X-rated and deals with sex and reproduction. There are, however, no pictures, so you’ll just have to use your imagination.
Here, if you actually want to do any research in this area, you’ll have to learn some basic ostracod anatomy and how to do micro-dissections to determine which ones are males and which are females. This turns out to be very important (maybe) in terms of the ratio of males to females. Here’s why:
1) There are some species in which males are absent and all reproduction is parthenogenic, i.e., only female offspring are produced. Here’s a disconcerting biological message for some of us and one that dates back hundreds of millions of years–under some circumstances, males are biologically irrelevant.
2) There is another group in which males are always found and reproduction is usually sexual with occasional lapses into parthenogenensis.
3) A third group consists of species in which there are indeed males, but not in large numbers and reproduction is both sexual and parthenogenic.
4) Finally, there is a group of species in which males are quite infrequent and parthenogenesis is the usual mode of reproduction.
So, now it should be very clear that in order to satisfy your prurient interest with regard to ostracods, you have to be extraordinarily patient, systematic, and become very adept at micro-dissection. You remember that above, I introduced a qualification regarding the importance of the ratio of males to females. In 1935, a biologist names Lowndes published a paper titled “The Sperms of Freshwater Ostracods” which you can find in the Proceedings of the Zoological Society of London (1935) 1: pages 35-48. He argues that in spite of the complex reproductive apparatus of the male–which we’ll discuss in a minute–and in spite of the fact that copulation does take place in some instances within some species, that it does not result in fertilization and that furthermore the entire process has become an empty ritual rather like the ancient Aztec sacrifice of virgins to appease the gods; only in this case, if Lowndes is right, the tables are turned and it is the male who makes the sacrifice for naught.
The reasons why these issues regarding the sexual characteristics of this tiny crustacean are so intriguing is that the male can be regarded as the “super stud” of the animal world sperm-wise. Male ostracods have, in both relative and absolute terms, the largest sperm of any know animal!!! Sperm size is, of course, not a function of the size of an animal; if it were, we would expect an incredible size from the sperm whale. (Sorry–bad pun! Which means it’s good.) The size of an ostracod’s sperm cell is about 10 times its body length which means that if we take a largest specimen of about 3 mm., it would be producing sperm slightly over one inch in length! Human sperm size ranges from about 25 to 50 microns–that’s only 0.000984 to 0.001968 inches! All of this makes one wonder why the ostracods haven’t taken over the planet. Super Studs of Earth–Beware! The Amazons have arrived. You see, it may be that ostracod males are utterly irrelevant and remember ostracods have been around for hundreds of millions of years. It is known that in certain other micro-crustacea and some species of rotifers as well, males are not required for procreation and in some species no males have ever been found.
So, you might well ask, how does an organism that’s only 3 mmm. long accommodate sperm that are 30 mm. long? Very ingeniously. The spermatozoa are housed in the seminal vesicle which curves and recurves in a convoluted fashion and connects to a vas deferens which extends almost around the entire inner edge of the shell to connect to the penis (actually, penes–there are 2 of them). Now, how could something this long circumnavigate such a circuitous path. Mother Nature provides even for ultimately useless males. At the other end of the seminal vesicle is an apparatus called Zenker’s organ which is connected to the testes. Zenker’s organ is a cylindrical structure which functions rather like a bellows or pump during copulation and forces the spermatozoa through the duct work and out the penes. And image, it does all of this with virtually no brain but, I guess, there are some who would say the same of the human male.
If Lowndes is right and all of the males are now obsolete, I can just imagine the French ostracods marching in protest, chanting “Viva la difference!” and the slightly inebriated German ostracods celebrating decadence by marching and interrogatively chanting, “Vas deferens?” (At this point, my students used to boo and hiss appreciatively.)
As you can understand, trying to get a glimpse of these extraordinary sperm cells requires enormous effort, skill, and luck. Just imagine your frustration if you were to sacrifice hundreds of specimens only to discover that you had found a new species in which males were non-existent or extremely rare. Such an experience might drive you to take up dentistry.
I’ve shown you an image of my intriguing little green ostracod which probably belongs to the genus Cypria. I decided to sacrifice one and it turned out to be a female, so now I’ll probably be ostracized for committing ostracocide. Even worse, I know it was a female because it had eggs so I could be charged with ovacide as well.
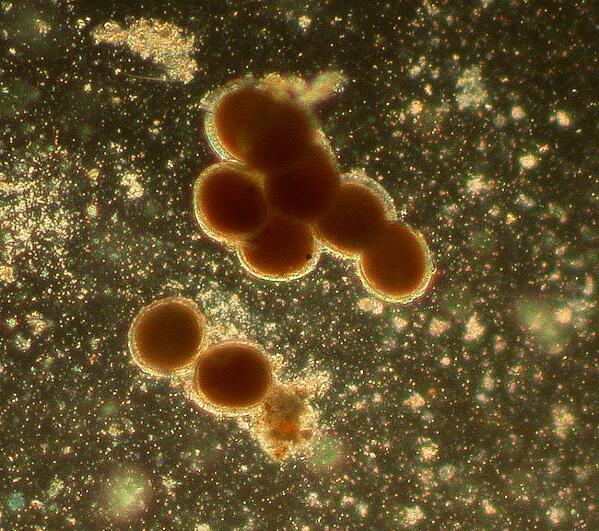
The central yolk, at this point of development, constitutes about 95 % of the egg, but if you look closely, you can see that there is a thin membrane enclosing the egg and a layer of clear material just below it.
Unsurprisingly, the eggs generally have a covering of calcareous material around them forming a very thin “shell”. I mentioned earlier that the valves of ostracods come in a variety of colors and some valves even have spots of a color different from the base color of the valve. As it turns out, the eggs come in different colors as well, but no spotted ones as far as I know. As one would expect, they are often white, but I have found orange and green ones and other researchers have reported yellow, red and pinkish ones as well. It is difficult to determine how many legitimate color variations there are, since the pink, red, and orange may just be variations at different stages of development. I seriously doubt whether there is any significant correlation between valve color and egg color.
Sometimes one will find just a single egg but, in my experience, it is more usual to find clusters on vegetation or debris. The eggs are highly resistant in adverse conditions and can survive drying for months or even years. I took about half of the mud sample that my friend brought me from Miletus and then divided that into 2 parts and placed each in a small culture dish to which I added some artesian water and a boiled wheat grain. After a week, I found some really tiny flagellates and some small hypotrichs.

These could have come from cysts in the air of my lab or from the wheat grains, but I rather think not. Today, a week later, I looked at the cultures again. I was a bit distracted because a friend of mine had called long distance and I was looking at the samples while talking to him. Then, after a couple of minutes, I was struck by the fact that these samples were full of little ostracods which had come as eggs all the way from the Temple of Zeus in Miletus, Turkey and had hatched! To me this was both wonderful and amazing and a further occasion to thank my thoughtful friend.
So, now let’s return to our local, green ostracod. The Turkish ones, by the way, are, so far, all light white in color.
Actually, the main thing I wanted to look at was the detail of the valve. As I mentioned to you before, the valves of dead ostracods which accumulate in the debris at the bottom of the sample lose the green color. What I discovered from closely examining some pieces of the valve was definitely ambiguous; in other words, I still don’t know what’s going on. Here’s why. You will recall that the valve consists of inner and outer mineralized plates with a thin layer of tissue between them. When the valve was crushed some of the green matter was pushed out into a blob away from the valve fragments which, however, also retained some of the green material, especially around the tuberculate edges of the valve.
So, here’s a picture of some of the green goo.
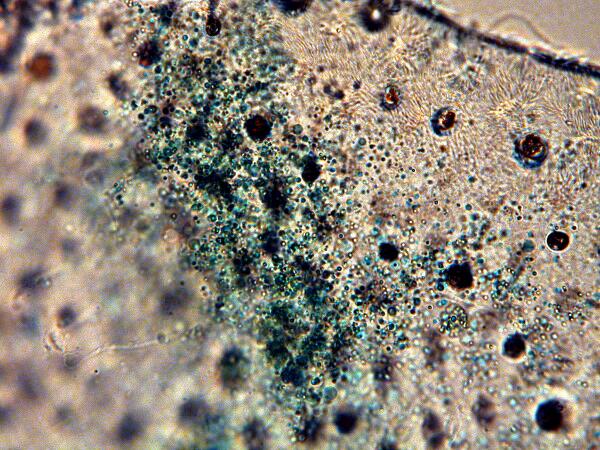
And again, at 500 magnification.

Here you will notice that the particles are extremely small and quite dark green. This doesn’t mitigate against their being very primitive algae or some kind of cyanobacteria, but it does complicate matters. These particles are in motion in the drop of distilled water which I added, but they are so minute that this movement could very well be Brownian motion. However, if the color is not organic, how do we account for it? It’s possible, I suppose, that these ostracods are able to extract some copper salts from the water and distribute them along the membrane inside the valve thus providing excellent camouflage among aquatic plants. After all, some ascidians (sea quirts) are able to extract the rather rare element Vanadium from sea water thus giving them green blood. This issue of pigment versus organic material in ostracods is probably a matter which only highly sophisticated micro-chemical analysis can resolve. Several investigators believe that the color is due to a granular pigment and their prime function is camouflage.
Very little work has been done on trying to culture ostracods in a systematic fashion. In part, this may be due to the fact that they can fairly readily be maintained in large jars or small aquaria that have a good productive layer of bottom detritus which will support a healthy growth of aquatic plants. It is, however, essential to limit algal growth by not placing the containers in direct sunlight or strong artificial light.
As stated before, specimens can be killed in 50% to 60% alcohol and stored in strong alcohol–85% to 90%. Formaldehyde tends to overharden the animal, and unless carefully neutralized, will attack the shells. Besides, formaldehyde is much more toxic and noxious to work with.
To study and/or attempt to identify ostracods, dissection is imperative. First, one must remove the animals from the shell which should be set aside for further examination and/or mounting. The ostracod should be placed on a glass slide and placed in a drop of glycerine. Ideally, the transition from alcohol should be in stages–50% glycerine, then after ½ hour pipet off the solution and add 75% glycerine, and after another ½ hour replace with 100% glycerine. You now have a nice clear, viscous medium for the dissection. I use micro-needles and micro-forceps. In an earlier essay, I described how to make both of these using the very small and extremely sharp insect pins called Minuten-Nadeln. Sometimes, a drop of stain is beneficial in helping to differentiate structures by providing additional contrast.
The appendages are taxonomically critical and so they should be dissected out and since they are already in glycerine, it is most convenient to mount them in a shallow cell of glycerine jelly and then the cell should be varnished several times to insure that it is tightly sealed. The valves can be dried and mounted in a cell filled with balsam or they may be mounted dry on a paleo slide with a circular depression as shown below.
In either case, it is desirable to mount one valve up and the other down.
The next time you’re sorting through a pond sample and come across some ostracods, don’t ignore them; they truly are fascinating little creatures.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .