Clear and Colourless Gems
by Robert Pavlis, USA
Diamonds
Carbon is the fourth most abundant element in the Universe after hydrogen, helium, and oxygen. Although less abundant in the Earth's crust than in the Universe at large, it is still anything but a rare element on Earth!
Diamonds are transparent crystals of carbon. Like many elements carbon has several different solid state structures that have been observed and isolated. At low pressures the stable form of carbon is graphite. Graphite consists of carbon atoms arranged in a sheet with each carbon atom surrounded by three other ones producing a flat sheet of fused hexagons. The structure closely resembles the hexagonal pattern of the hexagonal wire used for caging fowls. The layers can easily slip past one another, making graphite very soft, and, in fact, it is an excellent solid state lubricant. Carbon has four valence electrons and only three are used to form this frame work. The remaining electrons form an electron cloud that is half filled. A vast number of possible electronic excitations can occur within this structure so that all wavelengths from the near infrared through the visible and far into the ultra violet are strongly absorbed making this material black. The bonds are very stable, hence this structure is highly thermally stable. (However it will burn in air forming carbon dioxide.)

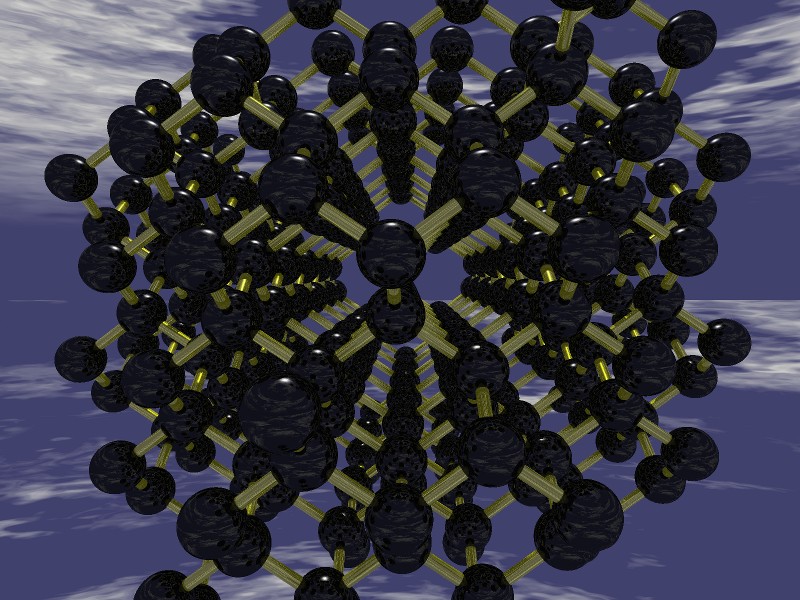
At very high pressure the stable form of carbon is diamond. Diamonds consist of carbon atoms each bonded to four neighbours with the bonds directed to the corners of a tetrahedron. On a larger scale there is formed an interlocking structure of puckered hexagons with ALL of the hexagons forming "chairs" similar to the stable chair structure of cyclohexane. The image above shows the diamond structure. (It was generated using two free and readily available computer programs, open Babel and povray.) From this image it is a bit difficult to see that this structure is face centred cubic. Cubic crystal groups are optically isotropic. (The index of refraction is the same in all directions, and there is no optical axis.)
The atoms are very closely packed together in this structure, only 154 pm to their nearest neighbours. This results in a density of about 3.52 grams/cm³ with carbon having an atomic mass of only 12.01 g/mole! The extreme density also results in an unusually high index of refraction—2.418 at 500 nm. All of the electrons of the carbon atoms are involved in single covalent bonds holding the structure together. A diamond is thus a single molecule!!!! There are no low lying electronic states so diamond does not absorb light by electronic excitation until sufficient energy photons are encountered to excite the single bonds—this takes ultra violet radiation shorter than about 220 nm. The spectrum is clear of absorbances from 220 nm all the way into the infrared.
Very few other atoms fit into the diamond lattice. However, both nitrogen and boron atoms fit very well! Neutral carbon atoms have 6 electrons, neutral boron atoms have only five. Placing boron into a diamond lattice results in electron "holes" in the structure creating a diamond p-type semiconductor. With the valence band now having vacancies electronic excitation becomes possible, and visible red and green light are strongly absorbed, making boron containing diamonds appear blue! A blue diamond is thus a diamond with boron impurity.
Nitrogen atoms have 7 electrons. Placing a nitrogen atom in a diamond lattice results in electrons being present above the valence band that can now be excited by light. This creates n-type semiconductor material. It turns out that these electrons strongly absorb visible blue light, causing nitrogen containing diamonds to be yellow or orange. A yellow or orange diamond is thus a diamond with nitrogen impurity.
Diamonds are extremely hard. They are often given credit for being the hardest substance known, however, this is not strictly true, a form of carbon made by crushing the "foot ball molecule", buckministerfullerene, C60, under pressures of about 40 kilo bars is slightly harder. Calculations and extrapolations also imply that a structure made by crushing graphite at moderate temperatures that also consists of tetrahedral carbon atoms, but with alternate atoms bonded covalently to the plane of atoms above and below the rings (Lonsdeleite) would be harder than diamond if it could be prepared or found pure. However, both Lonsdeleite and "crushed fullerene" are only marginally harder than diamond.
When diamonds are hit, sharp blows along planes corresponding to octrahedral faces of the unit crystal cells they can cleave cleanly. Diamonds are thus not as robust as their extreme hardness implies.
When diamonds are heated in air they ignite and burn forming carbon dioxide. When diamonds are heated to high temperatures in the absence of air they convert to graphite.
Diamonds are fairly rare on the Earth's surface because graphite is the stable form of carbon unless the pressure is extremely high—20 kbar or more. The pressure at which diamond becomes the stable structure is also temperature dependent, higher temperatures require higher pressures to favour diamond. The temperatures and pressures inside the Earth become higher and higher at greater depths. At about 150 km beneath the surface the pressure is great enough to favour diamond. The diamonds that are found on the Earth's surface are generally the result of unusual volcanic eruptions that bring material to the surface from great depth very quickly before the diamonds can convert to graphite. These conditions are met in the Kimberlite pipes of South Africa. There are similar structures and related types of ones located in several other places on planet Earth. Because these geological structures are quite rare, diamonds are also rather rare.
Diamonds that are dug from volcanic pipes are often octahedral in shape. They vary in clarity and colour. Native diamonds are very interesting. However, most are only beautiful in the way that the pieces of broken glass resulting from vandals breaking windows are beautiful!
Although large native diamonds are extremely expensive, it is possible to purchase gram sized samples of millimetre sized diamonds for reasonable prices. The image below was obtained using a LOMO MBS-10 microscope with trinocular attachment fitted with a Canon digital single lens reflex camera. The average size of these diamonds is around 1 millimetre:

Until relatively modern times diamonds were mostly used as charms because of their extraordinary hardness. It was not just until after World War I that the now classic Brilliant Cut Diamond was developed by the Belgian Marcel Tolkowsky. Shaping a diamond in this manner results in loss of a substantial portion of the diamond's mass, but it converts it into a shape that provides a display of sparkling colours. A modern brilliant cut gem has 58 facets.
The modern sparkling diamond exhibits what is often referred to as "fire". The Index of refraction of diamond at 500 nm is 2.418. The index of refraction of all materials varies with wavelength, shorter wavelengths have higher indices of refraction. This phenomenon is referred to as "dispersion." Dispersion is highly desirable in gem stones because it causes the diamond's sparkles to be coloured. Gemologists commonly take the difference between the Index of refraction at 686.7 nm and subtract it from the index at 430.8 nm and call this value the "dispersion" For diamond this value is 0.044. If one shine a point source of light at a brilliant cut clear gem stone in a darkened room one will see spectra projected all over the ceiling and walls of the room. The effect of sparkling colours is greatest in very bright light like sunlight because the eye's iris closes down so only a portion of these spectra enter the eye. Unfortunately the diamond industry has long given the world a propaganda barrage implying that diamonds are the perfect gem. Those who believe that almost certainly also believe in Santa Claus and the Easter Bunny! The truth is that diamonds have a problem: the dispersion of diamonds is only mediocre (at best). There are several other gem materials that have much better dispersion and hence exhibit much better fire than miserable diamonds!
Because diamonds have received so much advertising attention, materials with better optical constants than diamonds are commonly referred to as "simulants". It is not at all uncommon to find other clear gems than diamonds cut poorly to prevent their looking much better than miserable diamonds because jewellers find they can sell more of them if they look as bad as diamonds! Space aliens viewing the behaviour of earthlings in this matter would likely come to the same conclusion as Einstein did in his famous quote, "Only two things are infinite, the Universe and Human Stupidity, and I am not sure of the former."
Yttrium Aluminium Garnet
Yttrium aluminium garnet is a synthetic material that is commonly used in lasers. Techniques have been developed to permit the production of very large crystals of this purpose. Because the material is quite hard and very transparent it is commonly cut into gems.
The very large crystals that can be made of this material can be made colourless and defect free. Its hardness is about 8.5 in comparison to diamonds 10.0. That makes it hard enough to be used in jewellery with substantial resistance to scratching and damage, but it is inferior to diamond here. In its favour it is cubic.
"YAG" has an index of refraction of only 1.833. (As stated above, Diamond is 2.418). Its dispersion is only 0.028. It is not only mechanically inferior to diamond, the two optical constants that are important for a transparent gem are both strongly inferior to diamond.
In reality "YAG" is a poor material for jewellery, worse than diamonds and several other white gems.
Strontium Titanate (Tausonite)
Strontium titanate occurs as a natural mineral (tausonite), but natural specimens are small and not suitable for gems. Techniques have been developed, however, to produce this material in large crystals. Unfortunately much of the material today has a strong yellow colour. It is possible, however, to make it crystal clear and water white.
Strontium titanate has an index of refraction of 2.42, essentially identical to diamond. Its dispersion is amazing at 0.19. It has a cubic crystal lattice, so it is not birefringent. Its dispersion makes well cut specimens appear spectacularly better than diamonds. The "sparkle" and "fire" from a well cut specimen of this material is amazing. The diamond industry has always attempted to convince people that because it looked so much better than miserable diamonds that it looked "cheap." Incredibly they have been rather successful in convincing people that relatively ugly diamonds are worth more than large mansions!
Unfortunately, however, strontium titanate is NOT the perfect gemstone. Its hardness is only about 6.0 to 6.5. It also fractures easily. Thus the material with perhaps the best optical constants that has ever been found or developed has very poor physical durability, dramatically inferior to diamonds and almost all other competitors. Still it is still a wonderful gem material for earrings, tie tacks, and similar jewellery items that are not subject to strong impacts. If diamond had its dispersion one would find it much easier to justify the amazing prices that are often paid for diamonds.
Strontium titanate is not produced much today. This is partly due to difficulty of manufacture, and to the fact that there are other materials with far better physical properties that also are superior to miserable diamonds in their optical constants.
Cubic Zirconia
Around 1970 Russian physicists developed a method to prepare large crystals of zirconium oxide that had a cubic crystal structure rather than the thermodynamically stable monoclinic form. Zirconium oxide melts at around 2850C! The standard method of preparation involves placing several hundred kilograms of purified zirconium oxide, some metallic zirconium, and some yttrium and/or calcium oxide in a large water cooled copper container. Yttrium and calcium stabilise the cubic crystal structure.) This huge mass of material is irradiated with about a megawatt of microwave power—equivalent to the power of about 800 microwave ovens! The material melts, and a thin layer of unmelted zirconium oxide remains between the extraordinarily hot molten zirconium oxide and the copper container. Because zirconium oxide is a poor heat conductor and because the heat is being carried away from the container with the water cooling, the copper container does not melt. When the material is cooled large crystals of cubic zirconia separate. These are cooled over many hours, and the huge crystals can then be cut into gems.
The material is normally very very transparent with virtually no inclusions, cloudiness, or other faults. Because the crystals are large gems can be cut from it the size of a person's fist! Large uncut crystals are amazingly inexpensive for such a wonderful material. (Price: about one hundred pounds or one hundred fifty dollars per kilogram!)
The index of refraction is somewhat variable because of variation in how much yttrium and/or calcium oxide was added, but is around 2.2. This is slightly inferior to diamond, but high enough to show nearly the same "sparkle" as diamond. The dispersion of cubic zirconia is around 0.066. This is enough better than diamond to make well cut cubic zironia look a bit better than the best diamonds.
The Hardness of cubic zirconia is around 8.5. It is fairly resistant to cleaving when subjected to sharp blows. It is not as hard as diamond, but still hard enough stand up to fairly serious abuse. It is MUCH more heat resistant than diamonds. Diamonds ignite a burn in air when heated to a few hundred degrees. Cut cubic zirconia stones typically sell for about 1/10000 the price of diamonds.
Much has been written about telling cubic zirconia from diamonds. Generally cubic zirconia gem stones are prone to have less inclusions and be more transparent, (but improperly compounded ones with incorrect amounts of Yttrium or calcium apparently can have serious problems. Diamond is an amazingly good heat conductor, cubic zirconia is a very poor one, indeed its production depends on this fact!
The fact that cubic zirconia is at once inexpensive and has better dispersion than diamond leads to an odd phenomenon. Cubic zirconia can appear in the lowest cost poorly made costume jewellery as well as in extraordinarily well made and beautiful jewellery. Sometimes people buy diamond jewellery and put the diamond in a vault and replace the diamond in the piece of jewellery with cubic zirconia to reduce the chance of loss, damage or theft!! One might ask why did such people not purchase the cubic zirconia jewellery item in the first place!!!
The fire of a large cubic zirconia gem is amazing. Some people obtain brilliant cut gems several centimetres on a side and use them for paper weights!
Silicon carbide (Moissanite)
Silicon carbide is a very rare mineral, and when it occurs as a mineral it is called moissanite. It can be produced very inexpensively by reacting graphite with pure silicon oxide at very high temperature, usually in an electric furnace. The material is widely used as an abrasive because it has an hardness of 9.5, just below diamond. The abrasive grade of silicon carbide is normally black from carbon impurities and could not be used as a gemstone. Sometimes some crystals are obtained in the industrial synthesis that are quite clear and that are near colourless. However, it is possible to sublime silicon carbide to make large generally colourless hexagonal crystals.
Since its hardness is 9.5, less than that of diamond, one might draw the conclusion that it is less robust than diamond. However, diamond cleaves relatively easily when subjected to strong impact along octahedral cleavage planes. Silicon carbide can shatter with strong impacts, but does not cleave along cleavage planes like diamond. Silicon carbide is dramatically more stable to heat damage than diamond. On overall robustness one would have to conclude that silicon carbide is MORE resistant to damage than diamond.
For a colourless gem stone the optical constants are critical. Unfortunately silicon carbide is hexagonal—not cubic. It is thus birefringent with two indices of refraction, 2.967 and 2.648. Both indices are higher than diamond. Because this material is hexagonal gems must be cut from large crystals in such a manner that the crystallographic axis is parallel to the gem axis or the gem will exhibit annoying double refraction. The material is extremely hard so it is, like diamond, difficult to cut into gem stones. However, its dispersion is dramatically better than diamond, but also dramatically inferior to strontium titanate: value 0.105. Specimens tend to have a faint yellow colour which is more apparent in day light than under tungsten filament illumination. The high dispersion, however, makes these gems generally look extraordinary.
The gem quality material is difficult to manufacture and cutting it requires special skills. For this reason it costs much more than cubic zirconia, but also much less than diamond.
The reality is, however, that moissanite is superior to diamonds in both robustness and in optical quality. Almost all the moissanite produced as gems is cut in the brilliant cut described above.
Microscopes and Gems
Ordinary stereo microscopes are ideal for evaluating gem stones for crystal faults and to examine the quality of cut. Diamonds and other transparent and colourless gems have quite high indices of refraction. This makes them look spectacular under microscopes. Because they are transparent they generally look their best with illumination from the side rather than with ring lights or substage illumination. Because most transparent gem stones are cubic most do not exhibit birefringence. Moissanite with its hexagonal structure is an exception to this.
Diamonds are notorious for having inclusions and other faults. Microscopic examination attends to make these faults painfully obvious.
Diamonds and moissanite are extremely hard and seldom show evidence of scratching. Strontium titanate is fragile, and gem stones of this material that have been used in jewellery tend to become chipped and scratched unless the user takes care to handle it carefully. Cubic zirconia is much more robust than most people believe, it is a bit harder than emeralds, slightly softer than sapphire and ruby.
It is very difficult to obtain good images of clear gem stones. Even images of diamonds and other transparent gems used in advertising never look anywhere like the actual gems! Transparent gems always look best when strongly illuminated by a single small diameter light source. It is best if the gems be held far enough from the eyes so that the little spectra projected by the gem's facets are spread out enough so that only small portions of the spectra enter the eye at once so that they appear to be coloured.
An interesting experiment is to take a large brilliant cut cubic zirconia gem stone (or for the ultra wealthy a 10 caret or more diamond!) into a darkened room and point a laser pointer at it. The brilliant cut stone results in many red dots all over the room. Next take a point source bright white light source. (An excellent one can be obtained by removing the reflector from a "Mag-lite" and inserting it into a wrapping tube such as used for aluminium foil.) Shine this at the gem, again in a darkened room. The room will be filled with little spectra project everywhere! (I have attempted to take pictures of this, and was not able to get any that did justice to the effect.) The "fire" one sees when observing colourless gems results from the eye intercepting one of these little spectra.
All comments to the author Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Published in the November 2010 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk.