|
If not otherwise stated
in the caption the microscopical pictures
were taken through the 100x HI objective.
It is
raining in Cancún. The rainy season has started and it is now
time to hunt
for ….. tree holes. Where a branch had fallen or has been cut; many
times a more
or less deep hole develops. It is a cup waiting for the rains.
For
eight
or perhaps more months the hole has received dust and leaves, or other
detritus
that was contributed by the wind, including the cysts and spores of
living
things. Now the rain fills in the hole with neutral or acid water, in a
city
were all natural water is hard. (Over
30
DH.)
It
is an
opportunity to see if there is something living in those special
microhabitats.
The
first
tree hole is no more than twenty meters from my house were a young
doubly branched
tree was pruned to allow only one stem to grow. With
time at 30 cm from the ground an oval hole with 25 x
15 cm diameter
has developed with a depth of 6 cm.

Fig. 2
|
Four days
ago it was dry with a consolidated earthy bottom and some small leaves
in.
Today
it
is filled with rainwater. And the water looks a little muddy and with a
superficial film of bacteria. It is an invitation to see the initial
fauna of a
recently flooded tree hole (fig. 2 above).
With my “sampler” (a little ladler) I took more
or less 50 ml of water, and a very little sample of dirt from the
bottom, which
I install in a 100 ml cylindrical flask for a couple of hours before
starting
the examination.
|
|
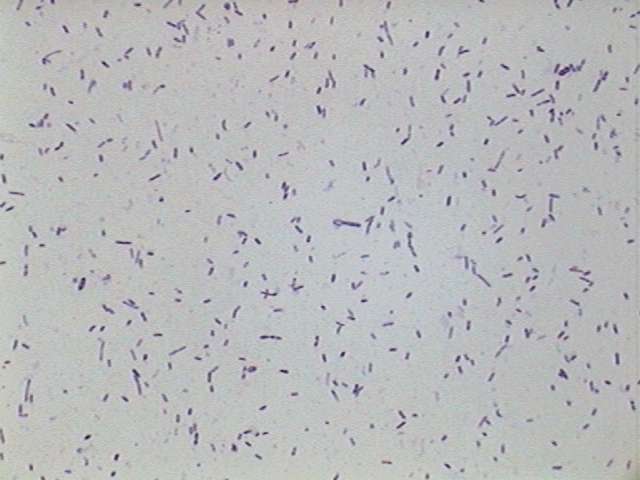
Bacteria
- stained with Gentian Violet.
|
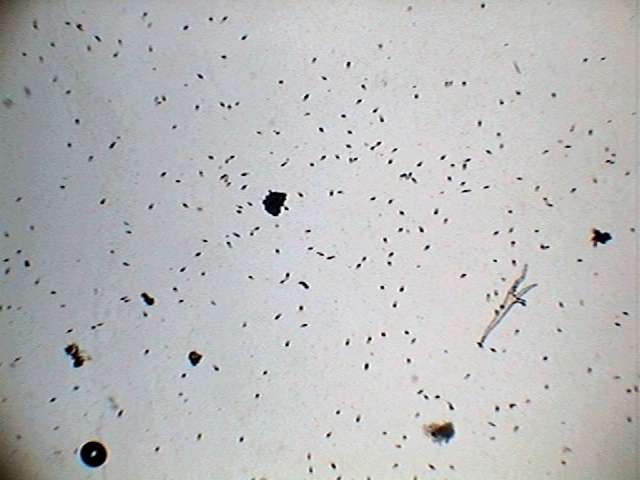
Astasia
- showing the density in a typical sample. Obj. x4
|
The
immediate exploration of the superficial and intermediate waters shows
plenty
of bacteria (mostly bacillus rods and some spirillum) and an
indefatigable
population of flagellates that swim at a velocity that made it
impossible
to shoot even
one in-focus picture. To show the density of the population I only had
to resort
to adding a drop of GALA, to
definitely stop the out of control
movement.
This
also permits a better understanding of its
morphology and permits us to assign the majority of the population to
an
euglenoid
flagellate of the genus Astasia.
LIVE EXAMINATION.
Astasia Ehrenberg 1838,
is a small
flagellate, of extremely
fast movement, which reached great densities in this culture. We can
see this
in fig. 3 taken with the 10x objective.
In
order to
see its morphology in more detail I must use at least a magnification
of
x1000
which requires the immersion objective, a very thin preparation and a
coverslide solidly fixed with a sealant. I used nail polish varnish.
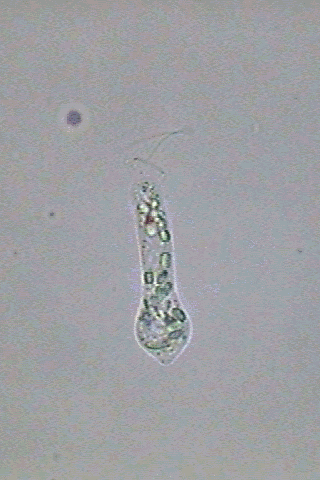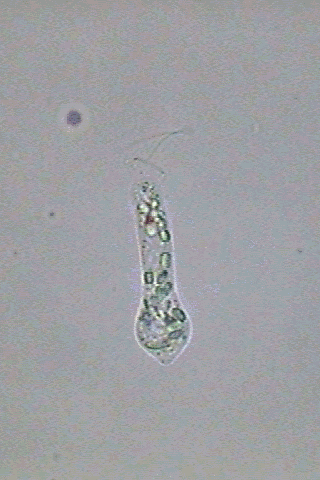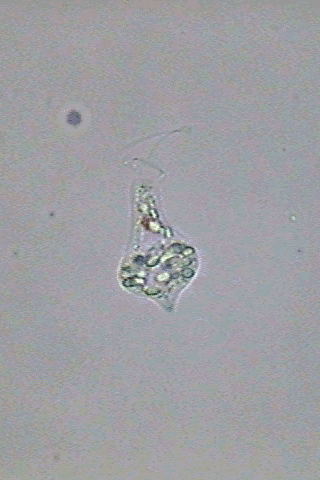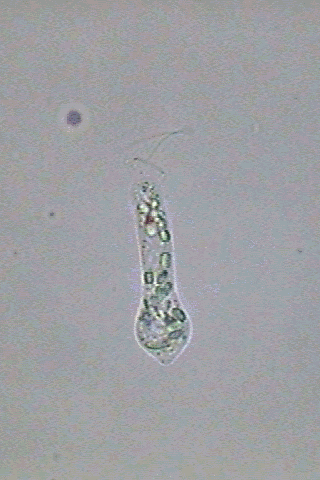
In
normal
swimming Astasia appears fusiform,
with the anterior
end more or less blunt, and an acute caudal end (fig 3).
In
the anterior end there is one short invagination of the pellicle
(canal, or reservoir)
erroneously denominated in English as the "gullet". When some euglenoid
species
ingest foods, (like Peranema, by ex.) they do it
generally with a
temporal buccal opening, or one that is permanent and
provided with reinforcing rods
(trichites)
always
placed outside and below the canal.
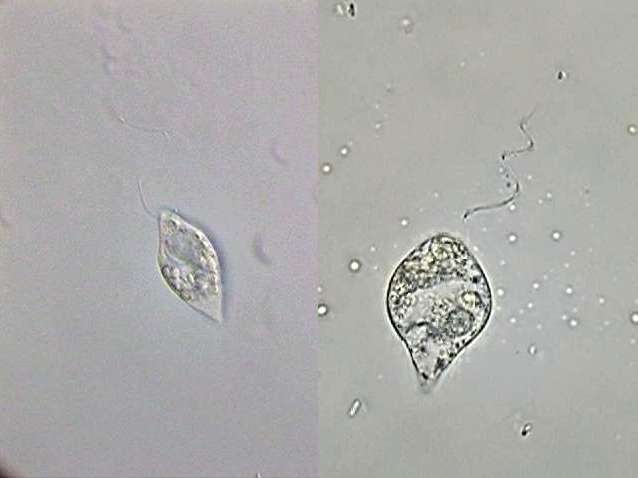
|
Fig. 3-Astasia
klebsii:
alive (at right) and a big specimen fixed with GALA at left
|
Instead
the
reservoir provides space and protection for the insertion of the
flagella.
Astasia species are
practically colorless, because they do not
have
chloroplasts like Euglena nor do they
possess euglena's characteristic red stigma generally found
in the base of the reservoir.
The
position of the reservoir allows the differentiation of two subgenera
of the
genus Astasia.
These are Euastasia and Euglenoidea. The population in
my tree
hole, by the apical position of the reservoir (fig 4)
and the flagellum, belong to the subgenus Euastasia
Christen 1963. In the
subgenus Euglenoidea
Christen 1963, the reservoir and therefore the emergence of the
flagellum is
subapical, as it occurs in Euglena.
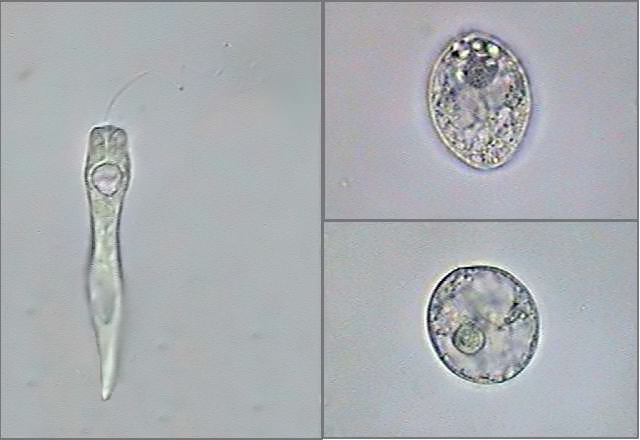
|
Fig. 4
-
Astasia
(Euastasia) klebsii -
extended and fixed individual, and two
pre-palmelloid states
|
Alive, the most
remarkable anatomical elements, aside from the flagellum are
the large contractile vesicles located next to the flagella
canal,
and the grains
of paramylon that tend to group itself at the rear. Paramylon
is a
complex carbohydrate that is easily recognized because it does not give
the
typical bluish black coloration of the starch, when tested with iodine.
The
individuals
that live in the substrate, or even some swimming ones, show a typical
movement
called "euglenoid movement" that consists of a local expansion of the
cell, shaped like a knot, which moves up and down throughout the cell.
Although
it is shown spontaneously in cells that have rejected its flagellum,
it's
easy to
initiate the euglenoid movement by adding to the drop with the
euglenoids
a very
small drop of fixative. Irritation causes the movement.
In
the individuals
with euglenoid movement the grains of paramylon tend to
concentrate
themselves in the posterior end, which in these cases is spherical.
The
nucleus
is only visible in favorable individuals, and it is not very often well
defined.
The size of
individuals varies of course with their contraction state,
from 30 to
40 micrometers in individuals in normal swimming (fig 3) up to 60-65
micrometers
in totally extended individuals (fig 4). The flagellum is at least one
and a
half times as long as the swimming cell, and the grains of paramylon
are
short
cylindrical rods barely 3.5 to 4.0 micrometers in length with a
diameter of
1.5 to 2.0 micrometers. The nucleus has an average diameter of 6
micrometers.
The
previous description and the captured images are consistent with the
species Astasia
klebsii Lemmermann 1910.
The
effect of asphyxia.-
if one leaves alone a sealed preparation for some minutes the
individuals react
to the lack of oxygen in a quite homogenous form. They shed the
flagellum, and
they adopt a shape that goes from an ellipse to a sphere. This is a
defense
state called palmelloid, that generally is completed by the secretion
of a
mucilaginous sheet, which allows the protist to subsist until finding
itself again in favorable
conditions. They accumulate paramylon in the posterior end and the
spherical nucleus is clearly visible in
preparations fixed with
GALA, with a large endosome and a granular content (fig.4).
Khawkinea Jahn & McKibben
1937
On
the
day of harvesting, the population was formed by myriads of small Astasia
klebsii.
But after three days in the laboratory, the dominance
switched
to a somewhat bigger species, wider and with a more tapered shape, Khawkinea
sp.
Khawkinea has a subterminal (lateral) reservoir,
in a position similar to the one of Euglena, and it also
has one red
colored ocular spot (stigma). Its only difference with the
euglenas is
that it does not have chloroplasts. In laboratory experimental
conditions Euglena
can be induced to shed its chloroplasts, and those individuals are
hardly
distinguished from a Khawkinea.
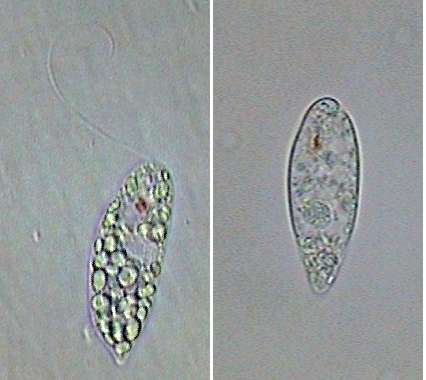
|
Fig. 5 - one Khawkinea
alive,
and one recently fixed. Notice the red ocular spot and the subterminal
position of the flagellum emergency point. In the left picture the
canal can be seen.
|
To
study
this species more easily I added to a 1 ml sample 10% of Rhode’s
fixative which induces the sedimentation of the suspended specimens, be
they
euglenoids or
ciliates, and gives them a mostly brown color.
The
length
of a typical specimen is around 45 micrometers, with a flagellum at
least
of the
same length, and even longer. In the fixed individuals the nucleus
measures 7 mic., and
a grain
of paramylon taken at random measured 5 x 3 mic.
An
individual stigma (fig 5, right) is seen in front view, and this
allowed the measurement of its
diameter which was 2.2 mic. As the stigma is always found at the bottom
of the flagellum
reservoir,
the measurement from stigma to the flagellum point of emergence gives
an
estimation of its length, which turns to be near 9 mic.
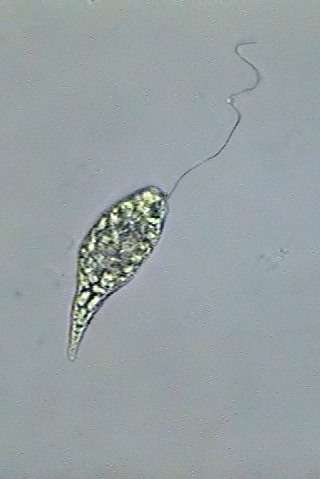
|
Fig. 6 - A Khawkinea, fixed
with Rhode's.
3 amalgamated
images to show the entire flagellum length.
|
As
seen in
the pictures the iodine present in the Rhode’s hasn't colored blue any
inclusion.
When
fixing
the specimens, or when these die by asphyxia they contract and adopt a
tennis
racket shape. Generally dead individuals have shed the flagellum,
but most
of Rhode’s fixed ones show it very well (fig. 6). The high density of
the grains
of paramylon
prevent the nucleus or the contractile vacuole to be seen.
Euglena
sp.
In only
one preparation appeared one lonely individual of a phototrophic
flagellate. It was a species of Euglena
of 100 microns
length, with small chloroplasts and cigarette shape. Perhaps it was the
announcement of a future change in the population.
Ciliates
from the surface.
At much
lower density (only one or two individuals to each two or three
preparations) appeared what I think are two species of Dileptus, a large and narrow one (Dileptus
sp.1), and another smaller but wider one (Dileptus
sp. 2). When little drops of fixative
were added
to stop them, the individuals rounded up and edematized. (fig. 7). Only
individuals fixed with
Rhode's maintained a
suitable morphology which allows them to be shown in a picture.
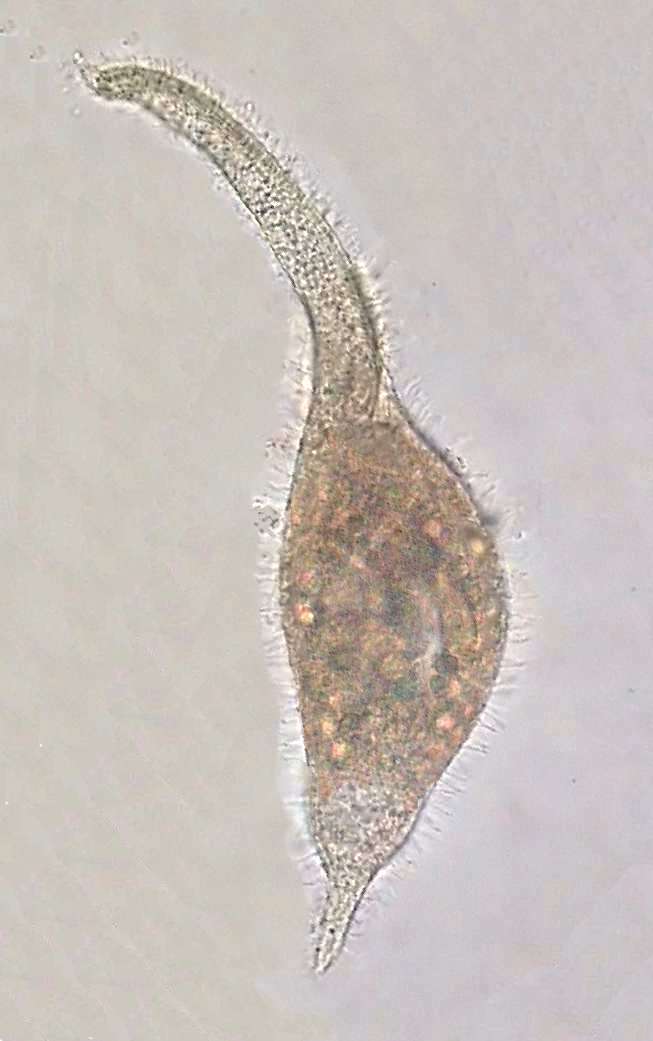
|
fig 7 - bad reaction to
anaesthesia intent
|
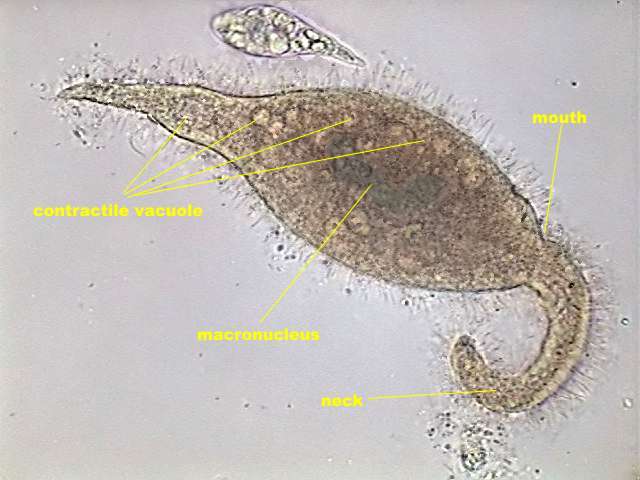
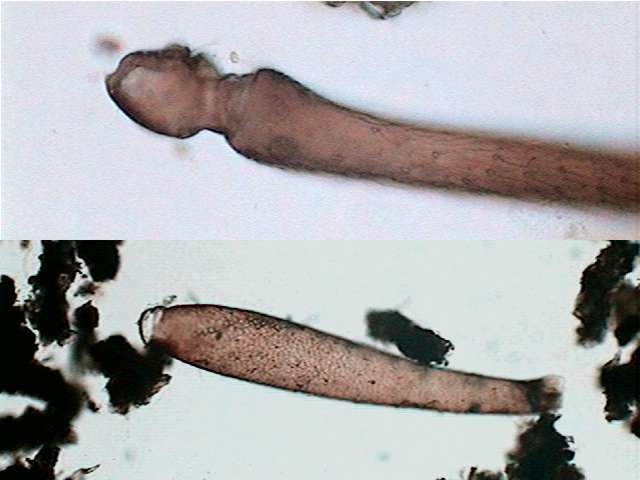
Dileptus moves
near the surface below the bacterial
veil. It swims impelled partly by his cilia, but extending its long
neck and waving
it in a helical manner (akin to the movement of a flagellum) while
rolling in
the water as they advance. This movement allows us to easily
distinguishing this
genus from Amphileptus, of very similar aspect, but of
shorter
and rigid neck. In the base of the Dileptus neck a
great mouth is
opened,
oriented forwards, circular and reinforced by trichites that turn it
into
a distensible
funnel.
|
The smaller but wider
species measures 200 to
250 micrometers of length with a maximum width of 50 to 58 mic. In the
photographed individual the neck represents 32 to 40% of the total
length.
In
the longest
and narrowest species the corresponding measures were: Total length 330
to
360 mic,
maximum width 35 to 39 mic. Neck/total length 40-48 %. The proportion
of wide
to total length was 10-12% in the narrow one and 20-24% in the wider.
Measurements were taken on pictures
of
live
individuals, swimming normally, that cannot be reproduced here because
the
movement of the protists doesn’t allow sharp enough images to be
captured. Both
species
have a thick and long macronucleus which seems to be built of two
sections slightly
displaced one with respect to the other.
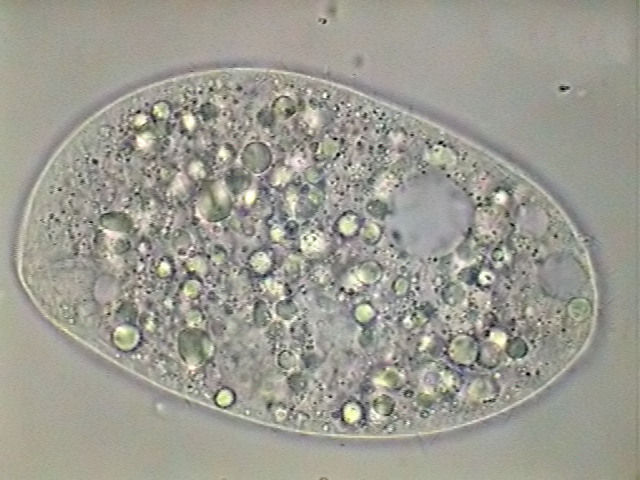
The
bottom and its inhabitants. The
accumulated sediment in the bottom showed it is composed fundamentally
by ejections of previous inhabitants. By its form and size they seem
to be faeces of some mosquito
species. (fig. 9)
Amongst them they teem Khawkinea, and also exists an
abundant
population of one small Vorticella with annulated
pellicle, and
with a hardly 40 mic. chalice length (fig.
10). By
its general
appearance, estriation and position of the nucleus it seems to be V.
cupifera
Kahl, 1935 (You can see
an
illustration in pag. 724, fig. 9 of Wimpertiere oder Ciliata, on-line
facsimile).
In
the
water immediately in contact with the sediment it moves with enormous
speed and
executes true zigzag jumps, (bouncing like a ball it is said sometimes to resemble)
one
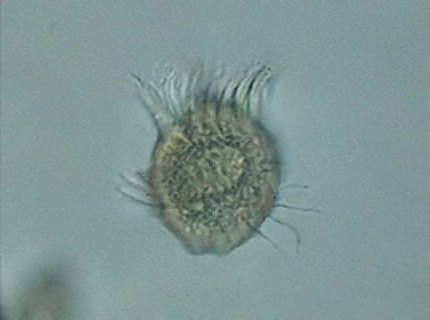
abundant population of Halteria
grandinella, a
spectacular small ciliate,
with a diameter not greater than the length of the flagellates.
|
| Fig. 8 - a big individual
of the Dileptus
sp. 2 |
|
fig. 9 - another Dileptus
sp 2, labelled
|
But
like Dileptus, this species is impossible to photograph
alive
without the aid of
an electronic flash. Nevertheless Halteria is fixable in
an acceptable shape by means of Rhode’s fixative. Although the
fixative
distorts a little the ciliation in fig. 11,
easily recognizable are the Adoral Zone of Membranelles, its Macronucleus and the Equatorial
Band of
Stereocils that allows its acrobatic antics.
|
|
| fig.
10 - detritus in the
bottom of the tree-hole. obj. x 4. Dark stop 12 mm |
fig. 11 - remains of
arthropoda in the bottom detritus.
x 4 obj.
|
Previous
inhabitants. Aside
from the evidence of the existence of dipteran larvae commented above,
they appeared
between the bottom sediments, fossil-like remains of other previous
colonizations,
a small Centropyxis and the remains of 2 bdelloids,
totally
lacking
of all content except for trophi. From the three fingers of the foot
and
the lack
of spurs it seems to belong to the genus Rotaria.
|
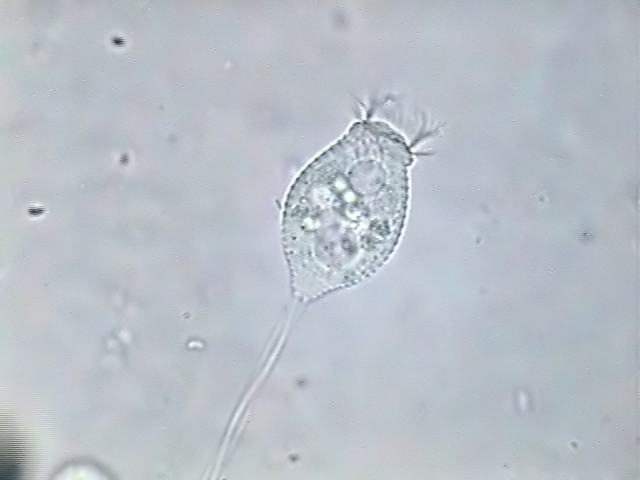
|
fig.
12 - Halteria grandinella. Fixed with Rhode's.
|
fig.
13 - Vorticella cupifera alive.
|
IN
SUMMARY
Four
days after its first filling, our tree hole of 15 x 21 cm with 6
cm of depth, supports an enormous and diverse bacterial population,
three
species of
flagellates and four ciliates. Both flagellates do not have
chlorophyll, neither
mouth, nor other elements for the capture of particulate food, and they
are known
to be osmotrophics.
Along with the bacteria, they exploit the
abundant dissolved organic and inorganic matter the rainwater extracted
from
the existing bottom sediments of the tree hole.
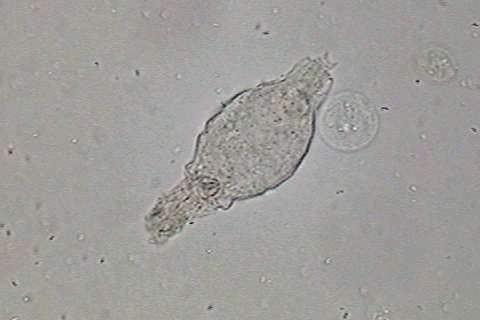
|
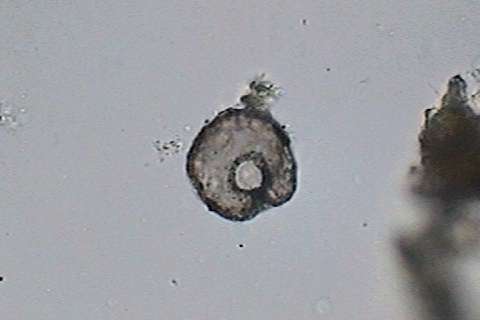
|
fig
14 - remains of a
bdelloid rotifers in the bottom detritus
x 40 obj.
|
fig.
15 - the shell of a
Centropyxis in the bottom detritus
x 40 obj.
|
Euglena is obviously phototrophic,
although it can also participate with the absorption of dissolved
substances, and
the ciliates are all heterotrofic. Vorticella and Halteria
they are bacteriophages and microdetritivores.
Dileptus is considered a predator. Being no other
visible prey we must consider that they consume the so abundant
flagellates and
Halteria. But I did not see them in any capture activity, nor do its
vacuoles
reveal digested Khawkinea remains.
Its enzymes must be really active.
Another
mystery of the tree-hole populations is the time at which reproduction
take
place. I have examined dozens of slides
all
day around but none at night. In none did I see any individual of any
species divide. But the populations of all of them are not only
thriving but
are
increasing.
|

