|
Caprice in C: Vitamin C Revisited by Richard L. Howey, Wyoming, USA |
Recently I got curious about how many articles I have written for Micscape, so I did a search and a count and came up with 95 essays. So, I decided that I would try to start producing something a bit special for the 100th article. Some time back, I wrote an article titled “Rhapsody in C” and promised a sequel, so this will be the sequel. Since I wrote the first essay, I have had some new ideas about a few preparation techniques and also some notions as to what other things I might try mixing with ascorbic acid solutions. My hope is to be able to produce some rather whimsical images–thus the title of this article–and maybe even cheat a little and add a bit of the Rothko magic elixir of Magnesium chloride.
My friend, Mike Shappell, a mathematics and computer science teacher, has commented to me on several occasions how there seem to be certain types and forms of patterns that the human mind appears to be programmed to find pleasing and that this is, at least in part, a cross-cultural phenomenon. Perhaps that will be borne out in some of the images which I have selected for this essay.
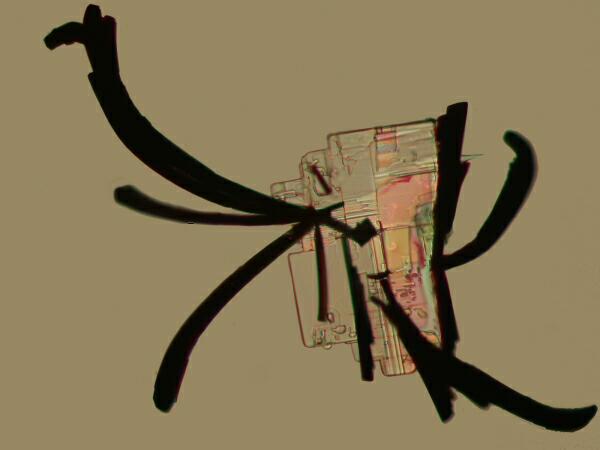
Again, I will be using polarized (or partially polarized) light as my means of exploring these crystalline labyrinths. For me, the delight of returning to these explorations is, in large part, re-encountering one of those hauntingly perplexing sets of borderline areas in nature where patterns and chaos seem to collide. As odd as this idea seems at first, if we observe and think about it carefully, it does make a special kind of sense in a framework we are not usually accustomed to dealing with. Let’s say that we mix some Magnesium chloride, which tends to produce rectangular crystals with Methylene blue, a common biological stain. I made several slides of this mixture and each of them showed some squarish crystals with black “feather-like” attachments giving the whole, the appearance of a bit of Chinese calligraphy.

Now, although similar forms appeared on each slide, they were certainly not the only crystal types nor were they usually even the dominant type. So, we do have definite patterns showing up as a consequence of this particular mixture, but we don’t understand why, because we don’t understand the local micro-conditions which pertain to those specific areas where these forms appear. Simply put, we don’t have a clue and we’re not likely to get a government grant to study such a problem when that government is more interested in manufacturing land mines, cluster bombs, thermonuclear devices, and other Weapons of Mindless Destruction. Ah, but we live in a new millennium with new priorities, so we’ll just have to try to puzzle these things out on our own.
Over the years, in observing not only my own slides and images, but those of others focused on Vitamin C tablets, I have observed several types of distinctive patterns that lead me say, at first glance,–Aha! Ascorbic acid. There may be other chemicals in the mix, but some of the forms are virtually a hallmark–you look at the image and you say to yourself-oh, yes, there is some ascorbic acid in here. Now, some of you less impressionable types might be inclined to say–but what’s so surprising about that? Crystals behave according to the “laws” of physics. Well, yes, and chemistry and fluid dynamics and biophysics and biochemistry as well, in some cases when one is dealing with organic systems.
My original interest in crystals developed very early on when I discovered that the spicules and spines of invertebrates I was fascinated by were either silica or, more likely, calcium carbonate. So, I began acquiring some specimens of nice calcite crystals and only too late learned that calcite has over 700 forms of crystallization. The naive assumption which I initially made as a young whippersnapper was that one substance would crystalize in one distinct form; this notion quickly evaporated once I began experimenting and if one stops to think about it, it is perfectly logical that this notion of multiple forms of crystallization would extend down to the micro-levels. In fact, it is precisely in this wonderful realm from 10 to 400 magnifications that diversity and pattern can reveal themselves spectacularly and often on a single slide!
Certain chemicals, ascorbic acid among them, have proved to be constant sources of surprise and perhaps this is one of the reasons that I keep returning to experimenting with crystals in new ways despite the fact that 2 or 3 of my friends have admonished me to concentrate more on organisms rather than crystals. To which I reply: I am studying crystals, so that I may better understand crystals in living organisms and the way they constitute skeletal structures. Well, it is fundamentally true and if I had another 750 years to devote to this enterprise, I think I could make some interesting headway. At one point, I did place some small sea urchin spines in one tube and a few sea urchin plates from the test in another tube and added a 10% solution of hydrochloric acid to dissolve the material which had been previously cleaned, washed, and dried. I had previously carefully examined the spines and plates under my stereo microscope to try to get an idea of the nature of the crystal lattices which comprise them. However, what I was curious about, in relation to this particular experiment, was how the crystals of the spines and plates once dissolved, would recrystallize. This experiment was initiated at a time when I was still teaching and, as was all too often the case in those days, I got interrupted and the tubes got set aside. Now that I am retired I, of course, have even more projects than ever before, but this little experiment intrigues me enough that I want to give it a couple of brief trials, hopefully before I finish this essay. If I find anything interesting, I’ll let you know.
So, back to the ascorbic acid crystals. Micro-crystals have long been not simply the subject of scientific investigation, but have appealed to the aesthetic dimension of our humanity as well and some photomicrographers have given their creative works highly imaginative and sometimes descriptive titles. I may do that here, but I warn you that you may find some ultramicroscopic whimsy involved. I am drawn to artists, such as Joan Miro, who had the delicious audacity to poke fun at the self-seriousness of his artistic fraternity by giving his works titles such as “Blond Woman Combing the Hair of her Armpit by the Light of the Moon”, but who can compete with that? So, I shall restrain myself and let you make up your own titles.
Yesterday, I started processing some images and so far I’ve got 160 images from just 11 of the 25 slides I’ve prepared so far and up until now I haven’t even tried any melts or “cocktails” or freezes or micro-wavings by way of getting some new types of results. Perhaps this caprice will turn into a full-fledged, four movement symphony. Just think of all of those billions of bits of colored electronic inks I would be using up. So, fair warning, I may yet have to write another 100 articles just on ascorbic acid.
For starters, let’s consider a few basic forms which, while not exclusive to ascorbic acid, nonetheless recur in quite distinctive ways and, importantly are virtually independent of any co-reagents; that is, other chemicals that I mix with the ascorbic acid. This is not to say that the co-reagents don’t have an affect; they do indeed and often in a manner that is also distinctive for that particular reagent.
Here are a few examples that are ascorbic acid-distinctive:
1) Concentric circles of “rings”. This is one of the loveliest and most striking features of ascorbic acid. These rings pop up in a variety of contexts and may, even under polarization, be essentially monochromatic but, far more likely, will be filled with vibrant, pastel colors. In imaging these crystals, it is important to remember that there is a strong correlation between illumination intensity and color saturation in the image.

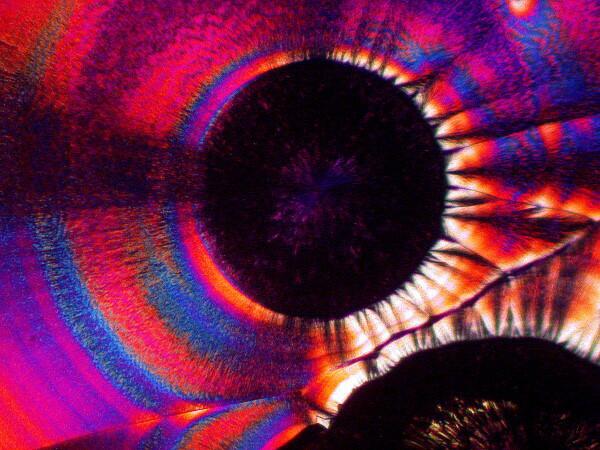

When I photograph with my old Olympus Stereo SZ-III I am able to use a small slide rheostat in my line cord to regulate the light intensity within a relatively narrow, but highly useful range. The color distribution can be altered dramatically by means of special compensators in a polarizing microscope (a rather expensive option unless you are doing critical crystallography) or by 2 very inexpensive alternatives (my options): 1) the use of full and 1/4 wave compensator films embedded in plastic. One can now buy these quite inexpensively in 2 x 2 squares. 2) This option is even cheaper; you can use the lid of a thin, small plastic Petri dish. If you wish you can even cut it to an appropriate size for your needs and then using a small, adjustable craft stand with a pair of forceps built-in, position the compensator in the light path of your microscope, thus permitting you to adjust the angle without holding the filter with your fingers.
For those of you who are epistemologically inclined, the question might arise: what are the “true” colors of these crystals? I shall resist the temptation to embark at this point upon a lengthy philosophical disquisition which I assure you would be more painful to me than it would be boring to you–well, alright, let’s call it a toss up. The simple answer is that it’s a bad question. First of all, it would depend upon the organism doing the viewing. Birds and bees, who were avid microscopists, would see the colors quite differently than we would and if you’re human and color-blind, take up tennis. We can of course, always establish sets of arbitrary standards and define the “true” colors of these crystals as those seen when the analyzer is at 90 degrees and the polarizer is at 0 degrees and there is no compensator or any other kind of filter being used and the color temperature of the light source is 3,200 K. So, sit back, relax, and enjoy the spectacle and stop worrying about trivial matters like “true and “real”–our politicians certainly have done.
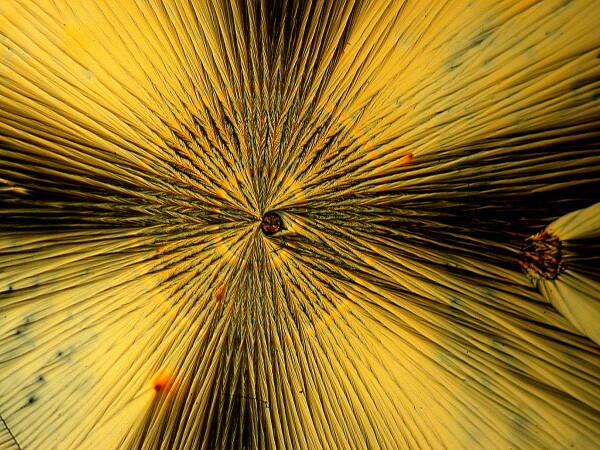
2) Cones. This form also occurs with some frequency and in a manner that is easy to identify as being associated with ascorbic acid.

Sometimes one can find an extensive cluster of such cones on a single slide.
3) “Eyes”. There are 2 basic kinds of eyes: a) The first type is essentially circular with a black center, although sometimes small crystals will form within the black area.
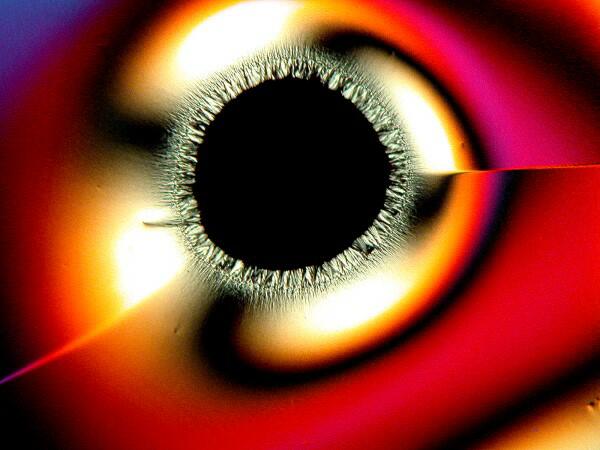
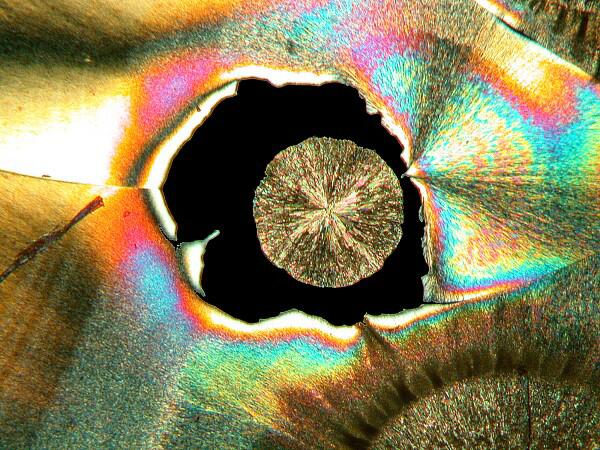
b) The second type is what I call the “feather” or “peacock” eye. The eye has the shape of an inverted tear-drop and then extending from it a rather long shaft like that of a feather.

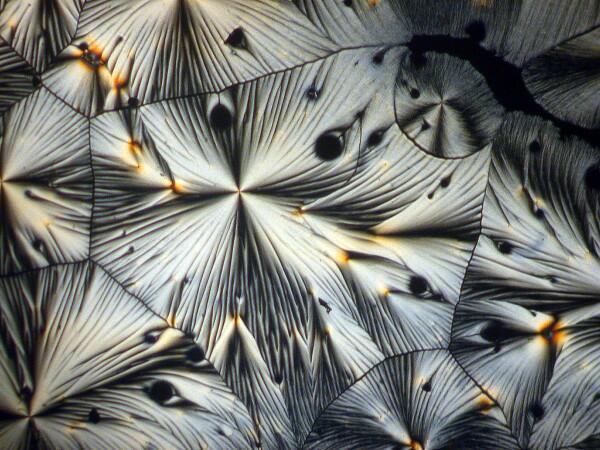

These are also quite common and can occur in significant numbers.
4) Feathers. Since we were speaking of feather forms, let’s consider another kind of example. Sometimes one encounters single shafts with small crystals radiating like barbules on a small feather shaft, but much more frequently one finds clusters of such shafts and very often they are arranged in a circular pattern radiating out from a center point.


5) “Coins”. Coins pop up in all kinds of places on a slide and some of them have a rich, gold color and so could equally be called ‘suns”.

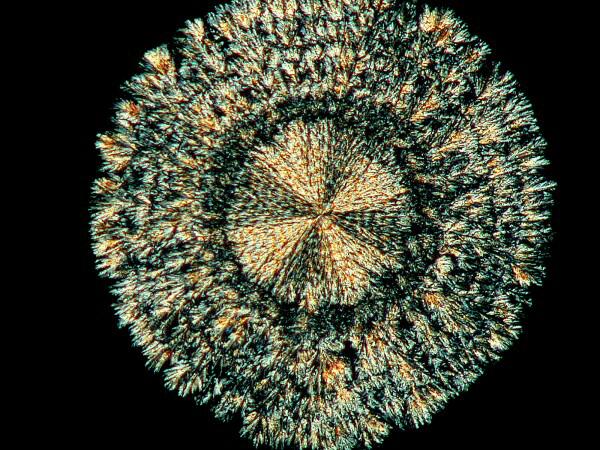
Sometimes you’ll find a number of them in a busy area where all kinds of other things are going on and then you’ll find isolated “anti-social” ones sitting off all by themselves. For some reason these coins seem susceptible to interesting modifications when mixed with certain other chemicals. Years ago, I was briefly prescribed chloral hydrate for my migraines, but I didn’t much like the effect and I had a bit of it remaining. If you’ve read my article “Leftovers”, you’ll know that I never discard such items which might later prove of interest and/or use in my lab. As it turns out, chloral hydrate modifies the coin-shape and “punches” small black holes in the coin.
Other chemicals produce what I call the “sand dollar” form which frequently has lovely pastel colors contrasting with the golden or silvery background.
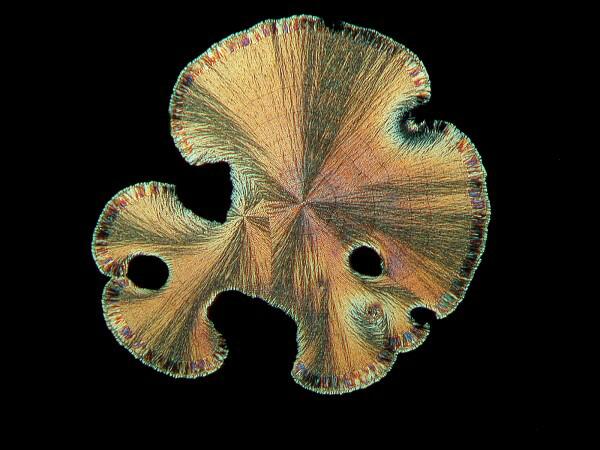

6) Textures. In addition to the issue of form, there is the consideration of a significant variation in texture. For example, if you look at the cone images, you get the impression that they are so smooth as to be polished and that if you were a wee microbe, you could go skiing down them.

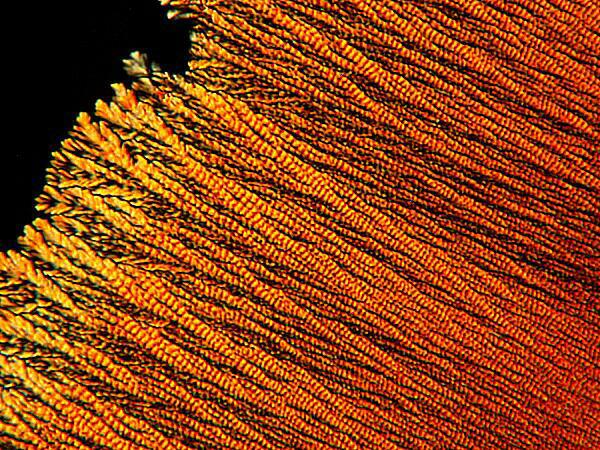
On the other hand, some reagents, such as a solution of iodine in potassium iodide and alcohol, produce a “ropey” texture in the filaments that compose a form.
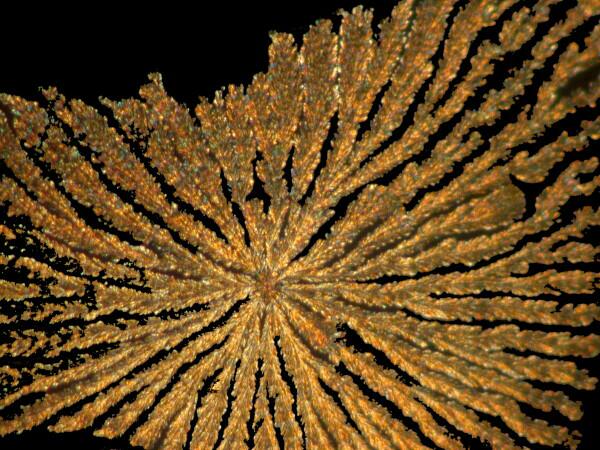

Yet other substances will produce a form which reminds me of the braided, rag rugs that my mother and grandmother used to make.
An interesting optical feature that occurs in a number of crystals, including ascorbic acid, is the so-called “Maltese cross”.

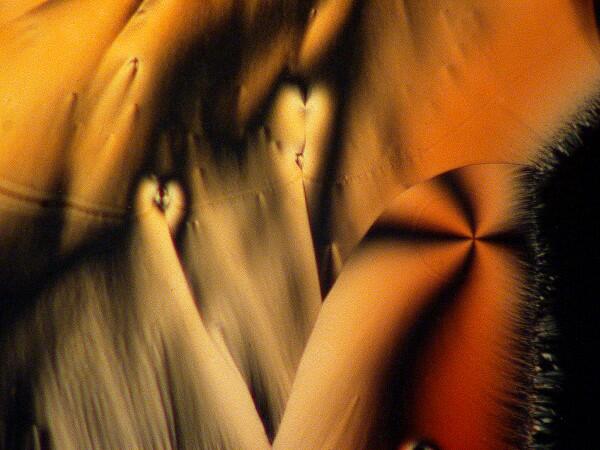
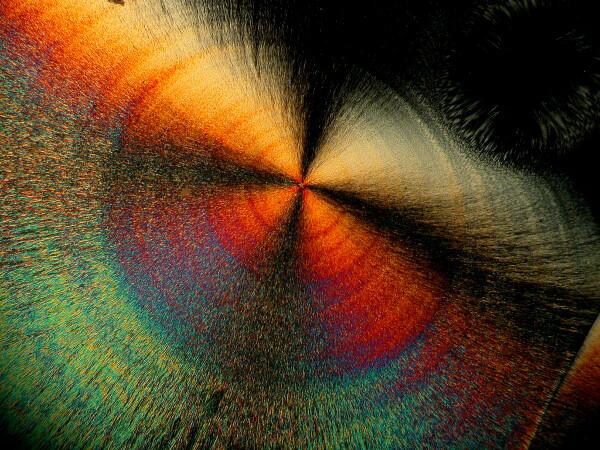
This distinctive feature can be found repeatedly once you begin looking for it.
Some Rather Odd Experiments
My wife and I love having fresh flowers in the house. However, after 8 years of drought, it has become increasingly difficult to maintain much in the way of any kind of garden. My compromise has been to buy cut flowers for which, fortunately, we have a source with an acceptable variety at reasonable prices. With each bouquet comes a small packet of white powder which is supposed to extend the life of the flowers. So, I got curious about what would happen if I made up a solution of this stuff–the packet doesn’t say what’s in it–and mixed it with ascorbic acid. I’ll give you 3 examples of what happened.
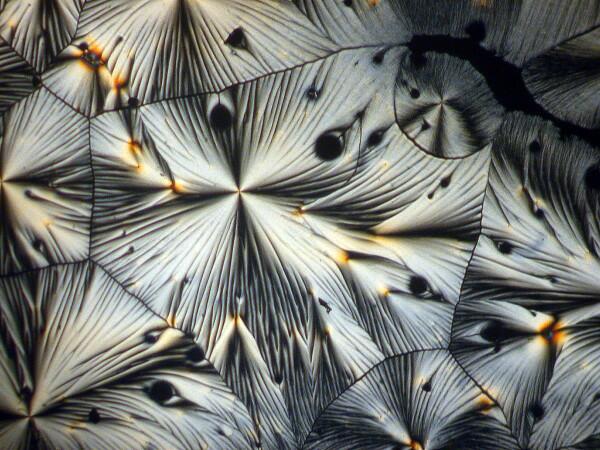

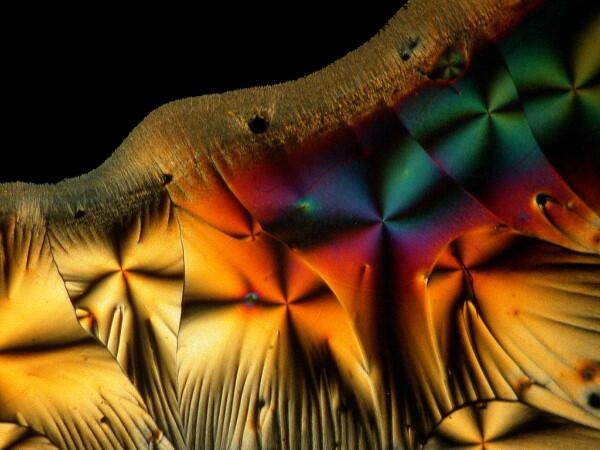
Hang on, it gets weirder. A few weeks earlier, I had sent a packet of forams to a friend of mine, but then I noticed one vial still sitting on my lab table. In packing I had overlooked it. So, I opened it up and scattered some forams across the slide and added a drop of ascorbic acid. Here are 2 results.
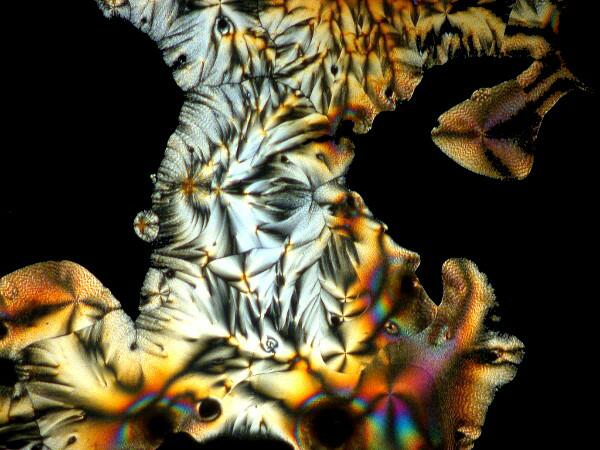
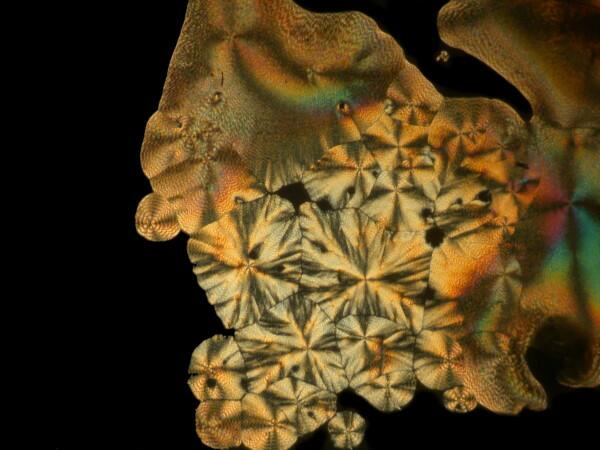
Last week, I was unpacking some dried marine specimens I had gotten from the Philippines and I noticed 3 interesting fragments still on my lab desk: 1) a bit of glass sponge, 2) a small fragment of soft coral, and 3) a lovely, fenestrated piece of a lacy bryozoan. So, you guessed it; three more weird slides. Surprisingly, the glass sponge fragments, which are essentially non-interactive chemically with ascorbic acid produced quite “busy” patterns as you can see from the example below.
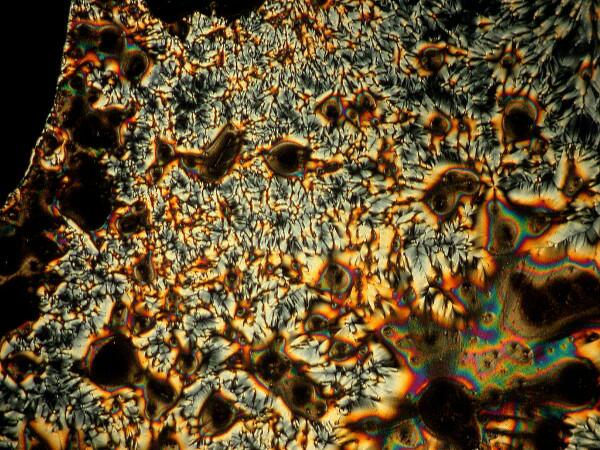
However, the real stunner was the lacy bryozoan. With your kind indulgence, I will overload you with ascorbic-bryozoan images. These are all from the first slide which I made and this afternoon, I made up two more slides and we’ll hope that they turnout to be lousy, otherwise I might be tempted to do yet another article on ascorbic acid eccentricities. As I was making up the slides today, I was thinking–O.K., I’m adding Vitamin C in solution to a fragment of the skeleton of a bryozoan. Why should it produce these results? So much of how we think about phenomena depends upon the language and the conceptual frameworks which we employ. Vitamin C plus a bit of a bryozoan skeleton–so why the reactions? When I made the slides today, I watched as I added the Vitamin C solution to the bryozoan fragment and noticed that it immediately began to bubble. Of course! I had been thinking in terms of an unproductive model. Vitamin C–an acid; bryozoan skeleton–largely calcium carbonate; naturally there would be a reaction and here we have some of the results.

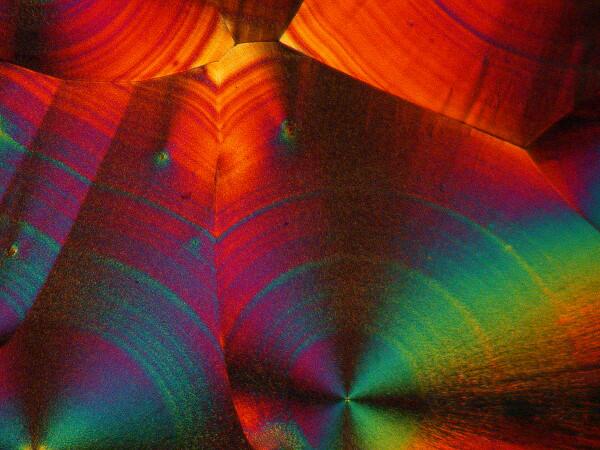
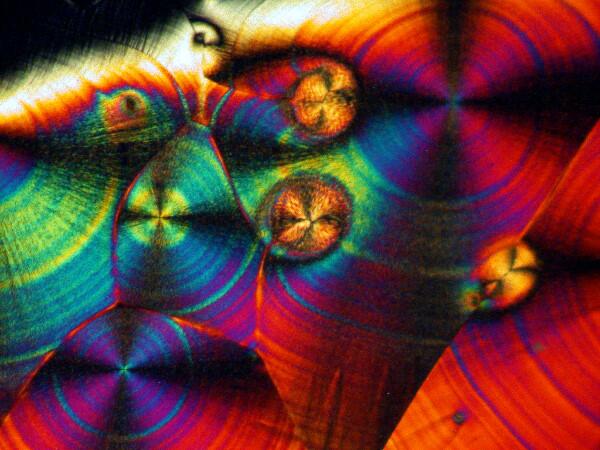
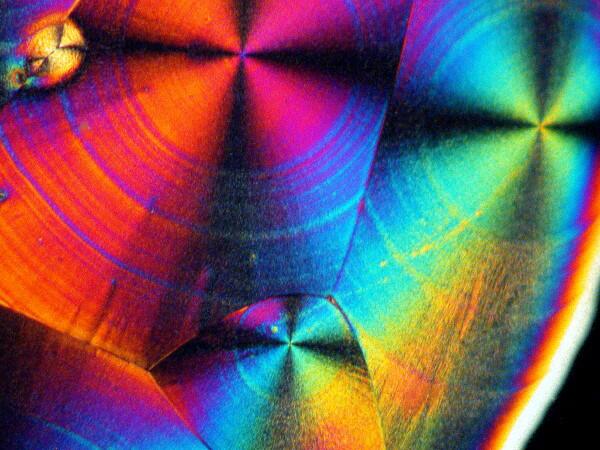

Recently, I have been studying sea urchins, some of which tend to shed their small spines readily and in considerable numbers during shipment. I have several little jars full of such spines, so naturally I had to try a few of them too. I made 2 slides using spines from 2 different species. Here are the results.
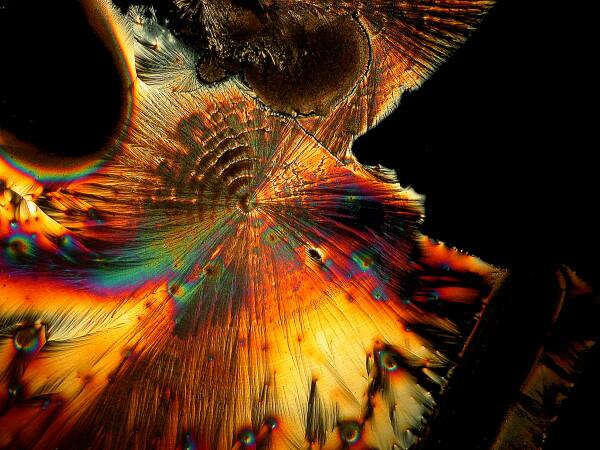
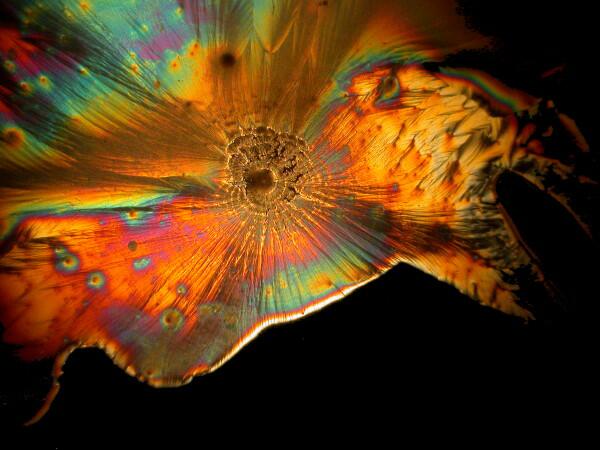
And just to show you how dull things would be without the wonder of polarized light, here is the same image without polarization.
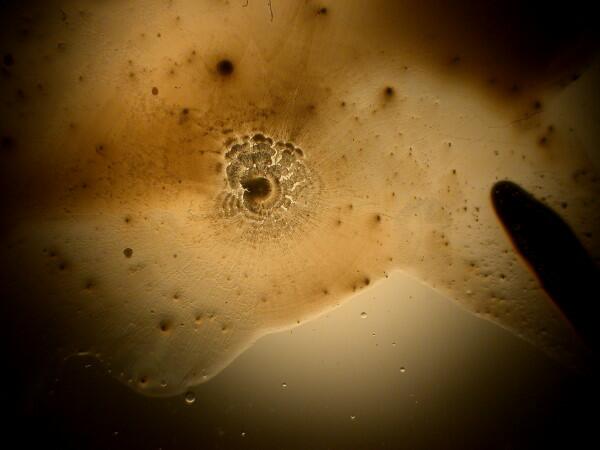
If you take some chemically “pure” calcium carbonate and add ascorbic acid , you won’t get these kinds of results. Sea urchin spines are composed primarily of calcium carbonate, but there are often traces of magnesium and strontium as well, plus salts from the surrounding sea water that have been deposited on the spine as well as vestiges of the organic membrane associated with the spine when the organisms were alive. There is also the additional complication of substances from other organisms which may be attached to the spine–sponges, gastropods, bryozoa, etc. In other words, the interactions between the ascorbic acid and the spines are very complex.
There is more, much more, but no room for it here. Nature provides us with a great series of endless adventure stories; ones which are often not easy to decipher, but the challenges which one encounters at one’s lab bench can be every bit as fulfilling as trekking down the Amazon–particularly for old fogeys like me who hate heat, camping, burnt food, and swarms of insects.
With all of the images I still have left, perhaps I shall have, after all, to do yet another essay on ascorbic acid–perhaps a Partita in C.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .