|
“I’m forever blowing bubbles” Part 1 An Eccentric Gallery by Richard L. Howey, Wyoming, USA |
Last year I was dissolving out some tissue from a chiton using sodium hypochlorite and hydrogen peroxide and I noticed the interesting pattern of foam which the oxygen bubbles made. Then more recently, I came across a splendid photomicrograph by the superb photomicrographer, Marek Mis, which showed bubbles trapped in a crystal melt of ascorbic acid. These two events got me to thinking about trying out some experiments using a variety of substances and examining them under polarized light. First, let me observe that this didn’t turn out to be as simple as I thought it might be. First of all, I no longer attempt doing melts of any substances due to a chronic pulmonary illness which necessitates that I use oxygen 24/7 and this means I can’t be around open flame or any potentially toxic vapors. Secondly, my initial attempts involved chemicals which for one reason or another were simply not interested in providing me any bubbles. At that point, I nearly abandoned the project, but then I recalled the results I had seen before and set about a second set of experiments which I am happy to say were more successful and provided me with a feast of new ideas for new approaches which I have yet to try. So, I hope this is the first of several galleries.
A substance that I use here for producing these images is a consequence of bad genetics and mediocre medieval dentists, namely, denture tablets. You pop one in water and it fizzes furiously. However, it’s necessary to take a tablet and reduce it to powder for the best effects. If you’re fortunate enough not to have to use such, then borrow one from your grandfather or buy a box of them at the drugstore; they’re quite inexpensive. All of these images were taken with polarized light and a quartz rotary compensator.
This first image is a combination of Stevia, the natural sweetener derived from a shrub, with denture powder.

The forms here remind me of the Spanish painter Joan Miro.
The next image is a mixture of the antihistamine gel form of Benadryl. Sometimes using a gel helps in terms of bubble formation. Also on this slide I used a circular cover glass; an edge can sometimes facilitate the formation of bubbles as well.
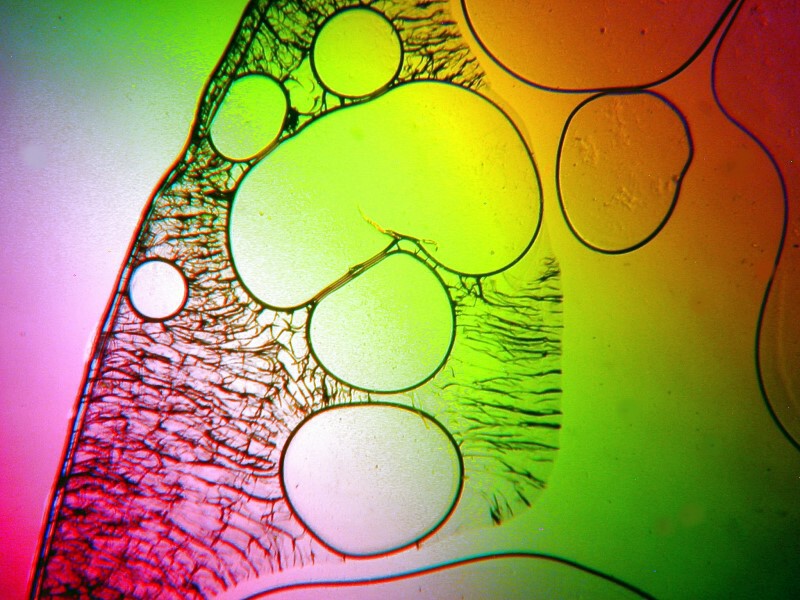
From time to time, I like to introduce a bit of material from a plant or animal into a mixture. In this case, I took a tiny fragment of a sea urchin spine and crushed it. In addition, there was denture powder and also ascorbic acid which activated both the powder and the spine fragment which is calcareous and you can see how the bubbles are forming around a bit of that spine.
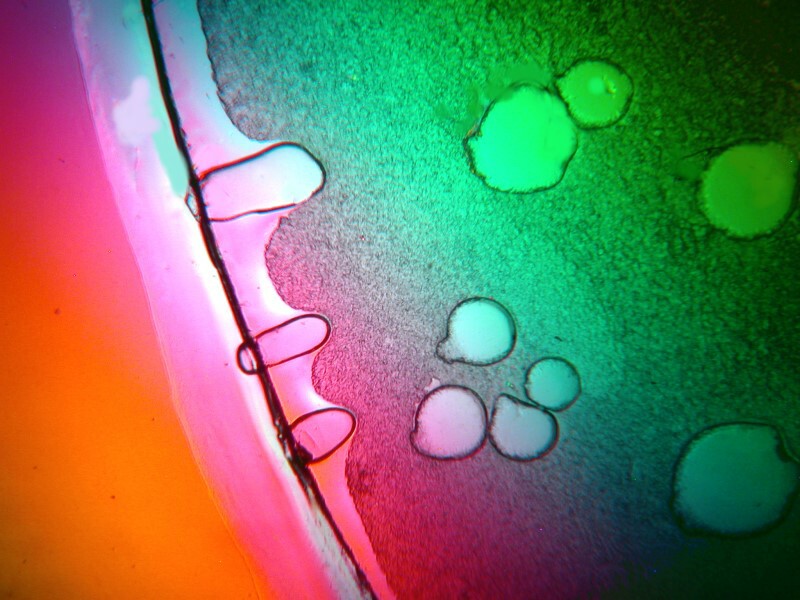
A mixture of urea, ascorbic acid, and denture powder produced an odd-shaped, vacuolated structure surrounded by wisps of birefringent material from the denture compound.

A very reliable substance for producing interesting polarized images is magnesium sulfate. I just buy the Epsom salts you can find in the drugstore or supermarket. I suspect that the impurities actually contribute positively.
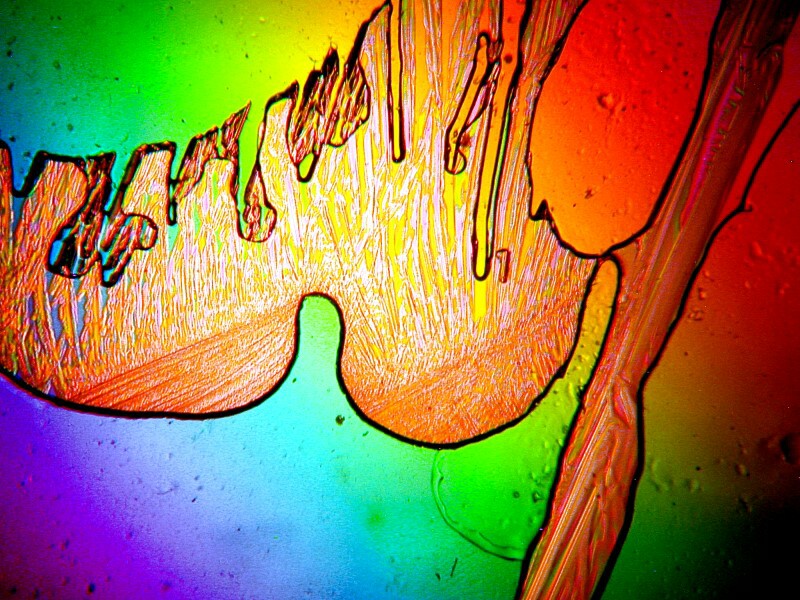
And just when you think you’re beginning to figure out how certain combinations produce particular types of patterns–ZAP, Mother Nature fools us again. I find ascorbic acid frequently produces disks and often with a ropy texture, so I didn’t expect this type of image below. I added another variable which produced effects that I didn’t anticipate. This was simply a combination of ascorbic acid and denture powder, but then, at the last minute, I added a drop of 70% isopropyl alcohol for the turbulence and, of course, turbulence turns out to be what is perhaps the most complex physical phenomenon in the known universe.

So, since I was bravely trying new combinations, I decided to introduce yet another variation; this time, ascorbic acid, denture powder, and hydrogen peroxide which is, of course, bubbly stuff in its own right. I was pleased with the results and will show you 3 images from this slide.
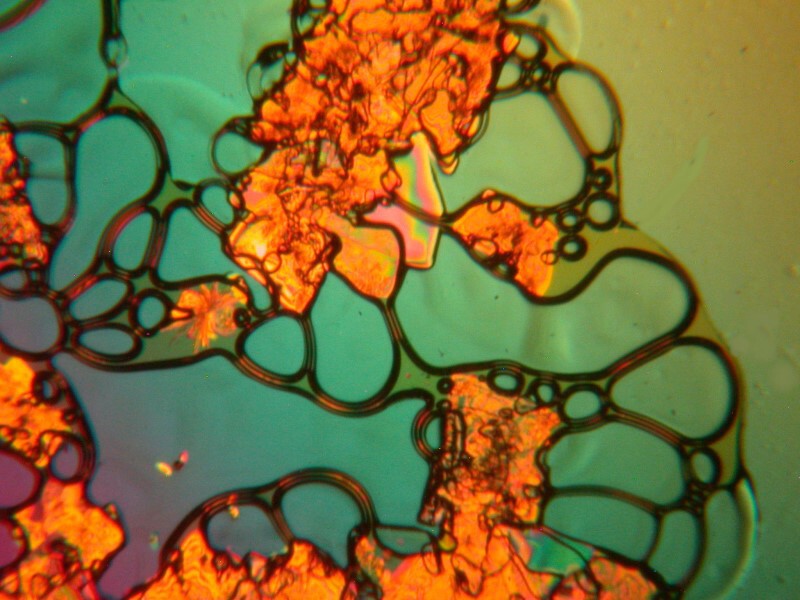
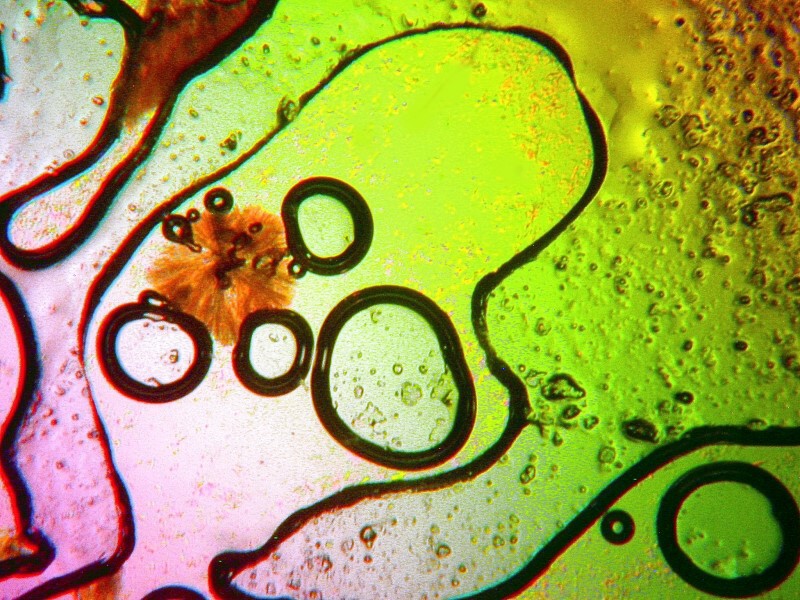
I especially like the double bubbles in the image below.
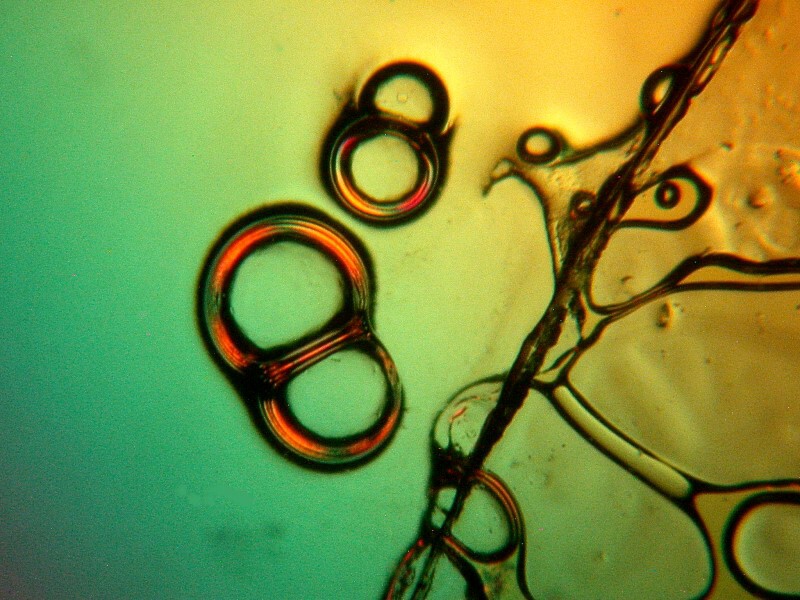
The chain below is also pleasing and here again we see the bubbles clustering around the edge of a cover glass.
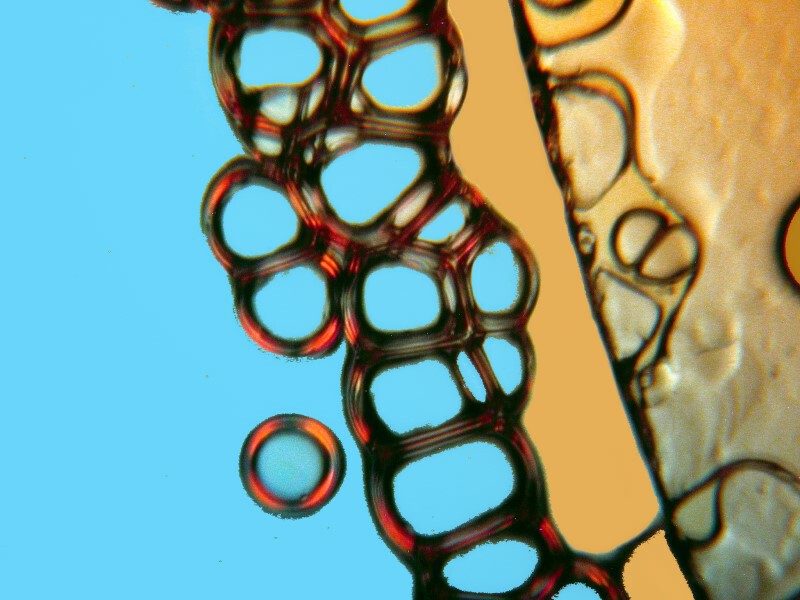
Clearly, the physical processes involved in the production of crystals are highly complex and, as a consequence, there is often a rather exciting element of randomness that enters in. However, there are certain factors that one can control and alter incrementally to move toward the kinds of results that one regards as desirable. Sometimes, just the combination of particular chemicals will give you surprising results which you can then later work on refining. I got some interesting images from a combination of urea, nickel sulfate, and denture powder. I’ll show you 5 of them without further comment.
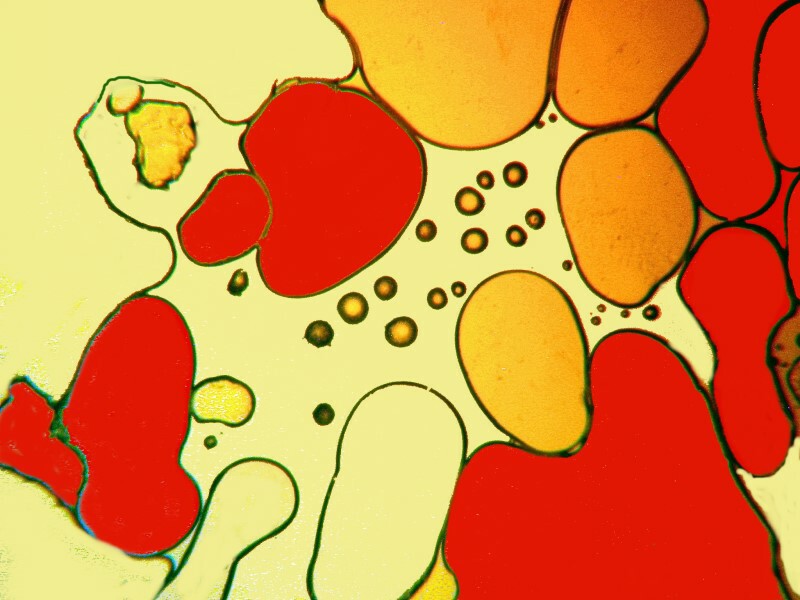
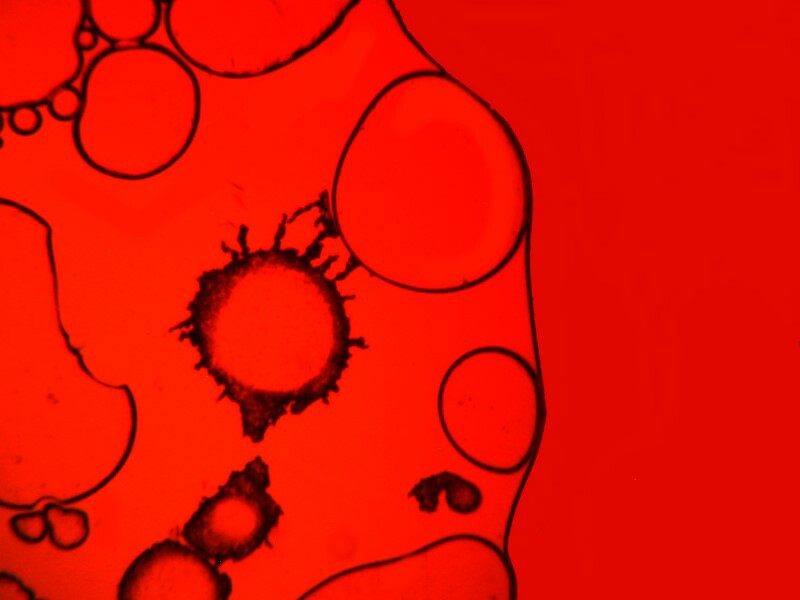
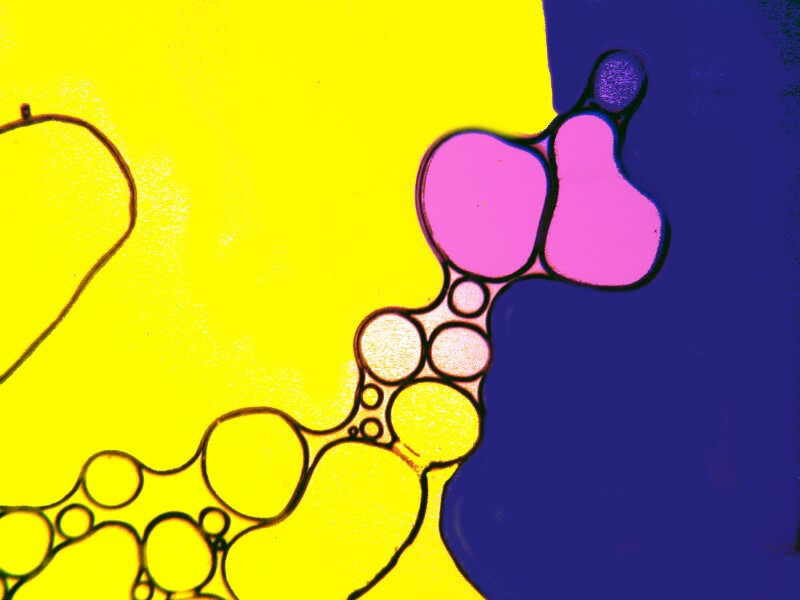
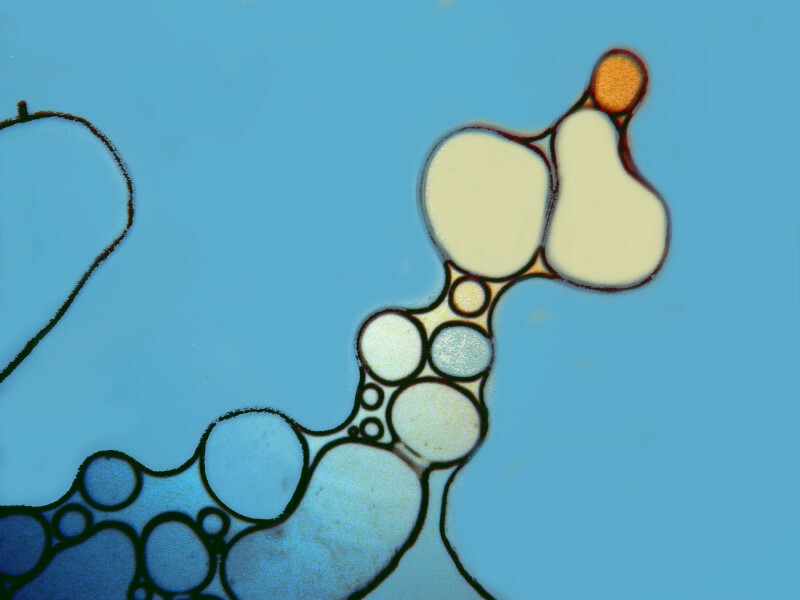

In the next installment, I hope to explore some other substance and techniques and try to achieve some additional variations on the theme of bubbles.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the October 2017 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .