|
|
A Gallery of Potassium Chlorate Photomicrographs KClO3 ( using polarized light and dark-ground illumination ) by Brian Johnston (Canada) |
|
|
A Gallery of Potassium Chlorate Photomicrographs KClO3 ( using polarized light and dark-ground illumination ) by Brian Johnston (Canada) |
Few chemical compounds bring back as many memories for me as potassium chlorate. As a secondary school chemistry teacher, I used the substance for both class lab experiments and demonstrations. In one experiment, students would place a small quantity in a test tube, and then fit a loosely packed glass wool plug into the open end of the tube. A Bunsen burner was used to melt, and then boil the compound. When a glowing wooden splint was held near the open end of the test tube, it would burst into flame. Those readers with a chemical background will know that oxygen gas is produced by the heated potassium chlorate, and this gas facilitated the combustion of the wood. A second test tube was usually prepared that contained a mixture of potassium chlorate and a small quantity of manganese dioxide. When the experiment was repeated, the burning of the splint was much more violent than in the first case. Manganese dioxide acts as a catalyst in the decomposition of the chlorate, and a greater volume of oxygen was therefore generated in a comparable time period.
Even though the experiments mentioned above were a part of the prescribed curriculum, I always felt apprehensive whenever students worked with potassium chlorate. This substance is a very strong oxidizing agent, and as such is very dangerous. Contact with some substances, (even at room temperature), can cause fires, or worse, explosions. There are a few documented cases where students have not placed the glass wool plug in place, and some glowing ashes from the splint have fallen directly into the boiling potassium chlorate, resulting in an explosion.
I mention the above as a warning. I do not recommend the preparation of melt specimens of this compound. Heating a small amount of potassium chlorate to its melting point of about 370°C is foolhardy unless you know what you're doing, and have the proper equipment.
Nevertheless, this article presents photomicrographs of several very carefully prepared melt specimens of the compound.
Potassium chlorate is a commonly used chemical in the manufacture of safety matches and pyrotechnics. It is a white crystalline solid.
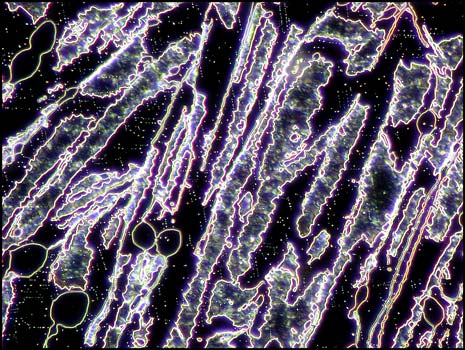
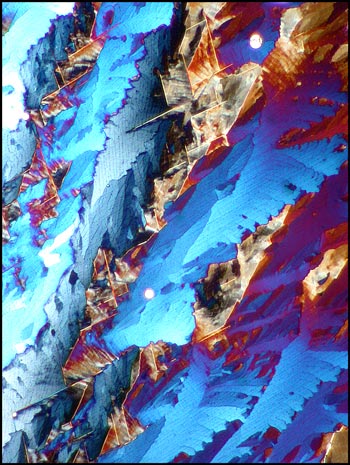
The first image in the article and the following two, show a distinctive characteristic of most melt specimens of the compound. Notice the sets of parallel fractures which sometimes form parallelogram shapes in the sample. These structures seem to occur on two different scales: one, on a larger scale which can be seen as light lines in the image on the left below, and one on a much smaller scale which can be seen as dotted finer lines in the same image.

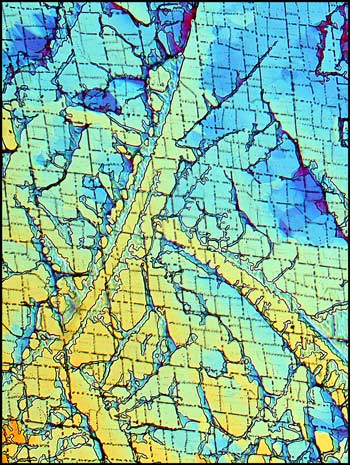
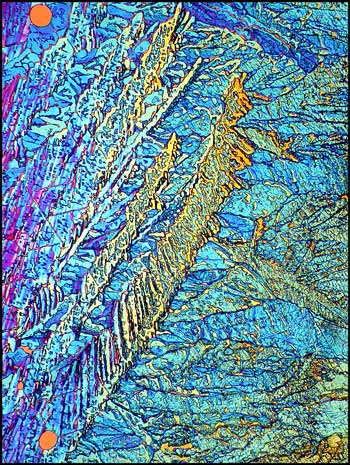
Crossed polars were used in all polarized light images, and compensators, (quarter and whole wave plates), were used to alter the colour spectrum in some images. The variations possible using this technique are many, as can be seen in the three images below of an interesting area of the slide.


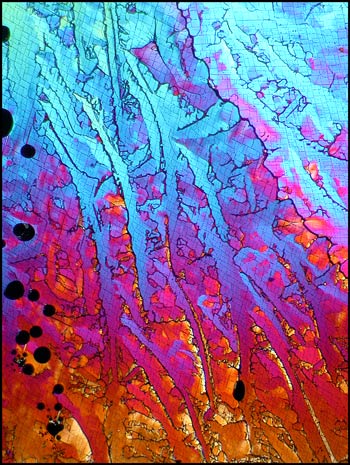
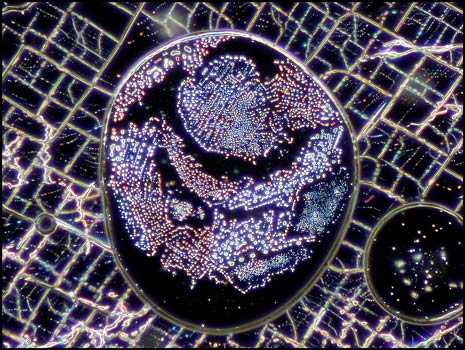
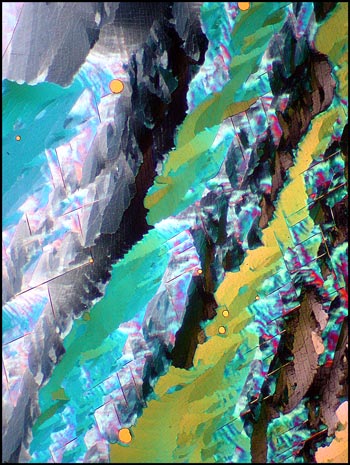
Many potassium chlorate melt specimens have bubbles which show as black in crossed polar photomicrographs. These blemishes can be seen in the two images below. As a chemistry teacher, I look upon the bubbles, not as an absence of potassium chlorate, but as the presence of small pockets of pure oxygen gas. As the compound is heated to the molten state (at 370°C), it is not difficult to bring the substance to the boil (at 400°C) at which temperature decomposition begins to take place. One of the decomposition products is oxygen gas. If instead of a Bunsen burner, a hot plate was used to melt the compound, greater control of the temperature could prevent this occurrence.

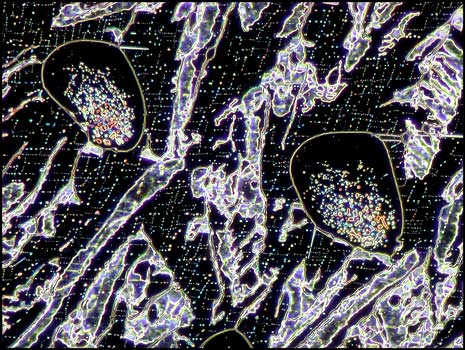
Dark-ground illumination provides a different view of these bubbles. In the first two images, some decomposition products have sublimed onto the slide within the gas bubbles. For some reason, the bubbles seen in the third image seem mostly free of solid material. Notice the parallelogram structures outside of the bubbles in the first two images.
°
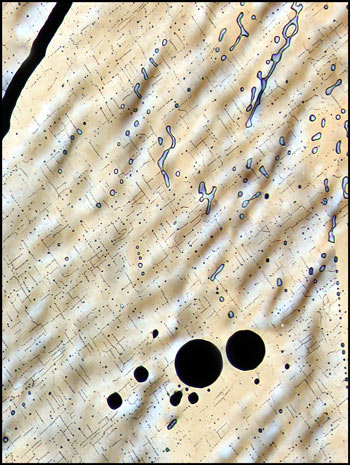
When part of the gray area in the above image is viewed at a much higher magnification, using phase contrast illumination, the result is shown below.
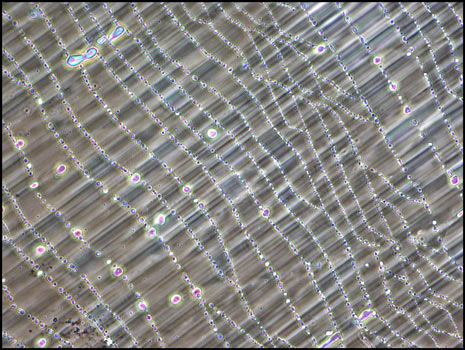
If a smaller quantity of compound is placed between slide and cover-glass, the resulting melt specimen is thinner. This results in a more pastel colouration of the crystals when viewed between crossed polarizers. (The bottom right image utilizes a compensator and for that reason the bubble shows up as yellow instead of black.)



The final three images are typical of this compound.
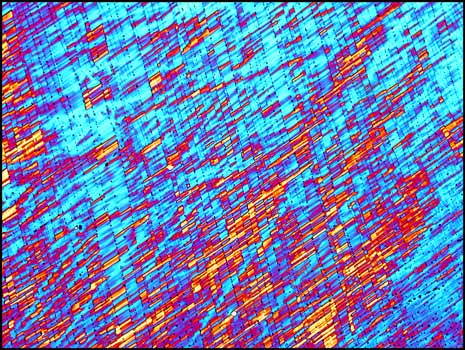
Most of the melt specimen substances that have been studied so far in this series of articles have been covalent compounds with fairly low melting points. Potassium chlorate is more ionic in character, and thus has a higher melting temperature, making it more difficult to work with. Although not well known to the general public, this industrial chemical is utilized in many diverse situations, from the burning of matches, to the oxidation of colour producing compounds in fireworks.
All comments to the author Brian Johnston are welcomed.
Published in the September 2004
edition of Micscape.
Please report any
Web problems or offer general comments to the
Micscape
Editor.
Micscape is the on-line
monthly magazine of the Microscopy UK web
site at
Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .