|
Nigrosin: A Different Kind of Staining Technique by Richard L. Howey, Wyoming, USA |
In examining protozoa, and medium to large ciliates in particular, one often gets curious about the surface features of the membrane which are not readily observable in the living organisms. Nigrosin is a fairly common biological stain which can be very helpful in such investigations. The tricky part here is not the staining technique, which is exceptionally straightforward, but selecting appropriate organisms to apply it to. The reason this is crucial is that one has to find protozoa that can be air-dried and yet retain their general form and shape and not undergo lysis. There is an alternative strategy which I will mention later.
Two ciliates which dry quite well are Paramecium and Euplotes . Place a drop of a rich culture on a slide, add a drop of Nigrosin, mix well, and serve–no, sorry, I’ve been watching too many old James Bond movies–mix well with a flat wooden toothpick and then use the toothpick to spread the drop thinly across the slide. You’ll find that not all of the specimens will give good results, but with a bit of patience and a few trials, you will get slides on which significant numbers of organisms dry intact and demonstrate intriguing surface features.
Consider 2 images of a Paramecium treated with Nigrosin.

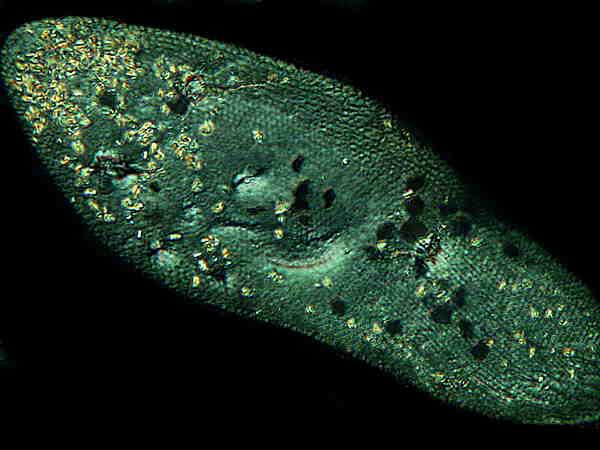
This image shows a specimen
treated with Nigrosin and photographed using Nomarski Differential
Interference Contrast (DIC). This technique uses polarized light
in conjunction with Wollaston prisms and polarization here shows
up the many minute crystalline inclusions in the Paramecium. They
appear as yellowish or yellowish-green spots and are most numerous
at the anterior end. The nucleus is clearly visible in the center
and below it and slightly to the right, part of the
cytopharyngeal basket or “gullet” is visible. Along the upper
edge, running from the anterior to the posterior, one can see a
series of more or less parallel lines which are the ciliary bands
or kineties.
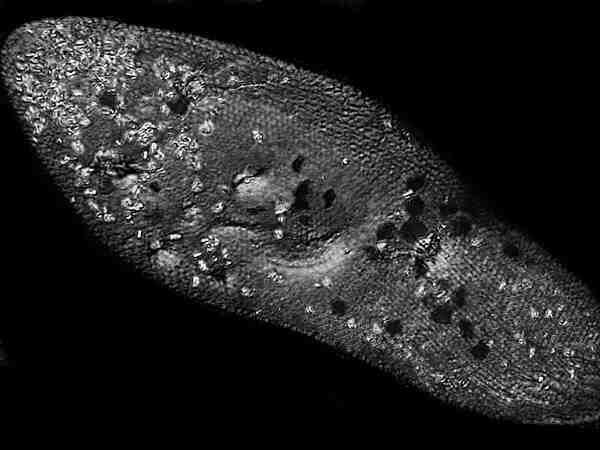
This is precisely the same
image which I altered with computer imaging software by using the
“monochrome” function. No other alterations were made. Here the
ciliary bands are clearer, but the crystalline inclusions don’t
stand out as dramatically as they do in the original
image.
Hypotrichs, such as, Euplotes also respond well to treatment with Nigrosin. Hypotrichs are a highly interesting group of organisms which, instead of great numbers of cilia, possess fused bundles of cilia called cirri which are evident in the image below.

This is again a DIC image. As you can see, the cirri are quite long–up to 1/3 the body length–and the protozoan can use them as legs and creep and crawl through the detritus on the substrate. However, hypotrichs are also strong swimmers. The dorsal membrane surface appears almost like a turtle shell and one can see half a dozen or so ridges giving the whole an armored appearance. Again, a few crystalline inclusions are visible.

This image is the result of
the application of the “monochrome” function with no other
alterations. This gives the organism a metallic appearance making
Euplotes seem like some creation for a sci-fi
film.
Concentration of the
Solution of Nigrosin
The only tricky part of
using this technique is finding the right concentration for the
organism you want to apply it to and this will vary somewhat. I
make up a stock solution of 10% which is much too strong to use
directly, but is convenient for dilutions. If the solution is too
strong you get a thin blackish-brown crust on the slide with
patterns on it and portions of this film will curl and peel. It
will also be sufficiently opaque that any organisms you had on the
slide will “disappear.” A good place to begin is with a 2%
solution which when you add a drop of culture of the same size
will give you approximately a 1% solution. If that’s not adequate,
then since you already have a 10% solution prepared, it’s easy
enough to make a 4% solution. Once you find a good concentration
for a particular organism, it’s a good idea to keep a detailed
record so that the next time you don’t have to go through the
trial and error procedure all over.
Some Other Experiments To
Try: A Challenge
As I was writing this, a number of questions occurred to me which I have not yet taken time to investigate. So, I’ll share them with you and the challenge is to see whether you research these issues and write an article on them for Micscape before I do.
1) Would this technique be of any use in revealing surface detail in the membranes of flagellates? I do know that some of the flagellates dry fairly well without major distortion, particularly some of the euglenoids. I also wonder if it might work with Volvox.
2) Would this technique be
of any use in revealing surface detail of the membranes of
amoebae? I don’t think that amoebae will dry very well for this
purpose which leads to the next issue.
3) Would it be feasible to use a fixative on some organisms and then apply the Nigrosin technique to study surface detail? This is a somewhat complicated matter, because adding this step could create artifacts by the Nigrosin interacting with some fixatives thereby giving false information regarding surface structure. My own suspicion is that if one uses a relatively simple formalin-based fixative without any metallic salts that might produce precipitates, then one might get quite good results. This could work well for a variety of flagellates and perhaps also for some of the tougher amoebae that are known to have textured surfaces, such as, the Thecamoebae and Chaos chaos.
4) I also wondered whether or not the Nigrosin technique might work for certain micro-invertebrates, such as, loricate rotifers and gastrotrichs. Some rotifers should provide very interesting images if the technique is successful and some gastrotrichs have an intriguing array of spines and scales.
I’ve already confessed to being lazy, so if I beat you and explore these questions before you do, then you are phenomenally, staggeringly, mind-bogglingly lazy and smarter than I am, since you get me to do all the work.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK