|
|
A Gallery of Cobaltous Nitrate Photomicrographs Co(NO3)2.6H2O (using
polarized light & dark-ground illumination) |
|
|
A Gallery of Cobaltous Nitrate Photomicrographs Co(NO3)2.6H2O (using
polarized light & dark-ground illumination) |
Cobaltous
nitrate hexahydrate, (or cobalt II nitrate hexahydrate as it should be
referred to nowadays), is most commonly used to supply cobalt ions Co+2
(aq) in water based solutions. These ions, (atoms with an electrical
charge), are used as a catalyst
in order to speed up the rate of many chemical reactions. The
hexahydrate term refers to the 6H2O in the formula.
Six water molecules are associated with each Co(NO3)2
group in the crystal lattice.
The red crystalline powder has the
extremely low melting temperature of about 56 oC, and this
makes it possible to prepare a melt specimen by placing a small
quantity of the solid on a microscope slide, covering with a
cover-glass, and heating gently over an alcohol lamp. As soon as
the solid melts and has formed a thin liquid film, the slide is removed
from the heat and allowed to cool slowly.
Note:
The MSDS safety document for the compound states:
Strong oxidizer - incompatible
with reducing agents.
Harmful if swallowed or inhaled.
Respiratory and eye irritant.
The substance decomposes on heating
producing toxic gases, including nitrogen oxides.
Occasionally, large almost perfect
crystals form amongst the general matrix of smaller crystals. The
two images that follow made use of elliptically polarized light. (Crossed polars + two lambda/4 plates)
One of the plates was rotated in order to produce the differences seen
in the two images.
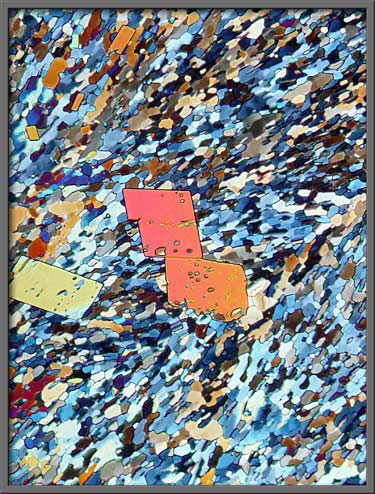
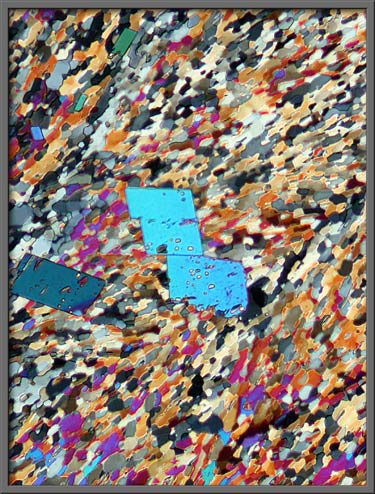
Sometimes the larger crystals are
far from perfectly formed, as the right hand image below demonstrates. (Crossed polars + two lambda/4 plates)


If the polarizing condenser is
replaced by a dark-ground condenser, the crystal edges are
highlighted. This produces a dramatically different view of the
structures.


Two photomicrographs of the same
crystal field can be seen below. Elliptically polarized light was
used to form the left image, while plane polarized light was used in
the right image. (Left: Crossed
polars + two lambda/4 plates - Right: crossed polars)


Low magnification barely resolves
individual crystals. (crossed polars)

A higher magnification reveals a
mosaic of individual crystals in the field. (crossed polars) Note: The first image in the article
is the same as the first image below, but it had Photoshop’s “Invert (colour)” command used on it.)


These mosaic-like patterns can be
striking in both form and colour. (First image: crossed polars – Second
image: Crossed polars + two lambda/4
plates – Third image: Crossed polars + lambda/4 plate + lambda
plate)
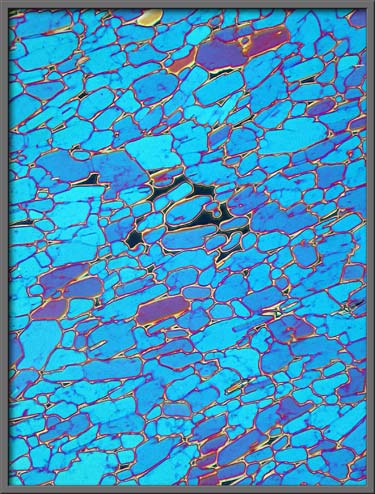


Here
is another example of the colour transformations made possible by
rotating one of the plates. (Crossed
polars + rotated lambda/4 plate + lambda plate)


The gaps between the (blue) mosaic
pattern are filled with random crystalline “garbage”. (Crossed polars + two lambda/4 plates)
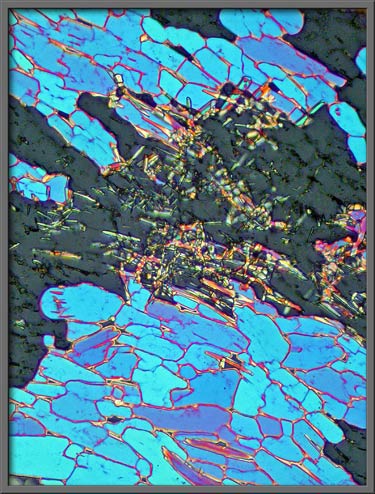
Cobaltous nitrate normally forms
long, thin crystals (monoclinic)
if they are allowed to grow freely without the constraints imposed by
melt specimen preparation. (Crossed
polars + lambda/4 plate + lambda plate)

Notice the interesting detail on
the light gray bridge feature at the center of the image below. (Crossed polars + two lambda/4 plates)

This same feature is shown at the
top of the two images that follow. Notice how the illumination
can accentuate or de-accentuate particular details. (First image:
Crossed polars + two lambda/4 plates
– Second image: Crossed polars)


As a chemistry teacher, I have
often used cobaltous nitrate as a catalyst in senior chemistry
experiments. Not only does the aqueous solution have an
attractive pink colour, but when the solid is melted and
recrystallized, the resulting formations are photogenic as well.
Photomicrographic
Equipment
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using two
illumination techniques: dark-ground, and polarized light.
Crossed polars were used in all polarized light images.
Compensators, (lambda and lambda/4 plates), were utilized to alter the
appearance in some cases. A 2.5x, 6.3x, 16x or 25x flat-field
objective formed the original image and a 10x Periplan eyepiece
projected the image to the camera lens.
Published in the
September 2007 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .