Robert Pavlis, Girard, Kansas USA
Often compounds that are interesting to study under microscopes are either difficult to obtain or they are hazardous materials. The sodium salt of the amino acid, S-glutamic acid can be obtained in grocery stores in pure form, and many people already have bottles of it in their kitchens. S-Glutamic acid is one of the amino acids that occurs in proteins. (In fact, it is the most abundant one in most organisms.) Glutamic acid is also one of the amino acids that has other rôles in metabolism.

Glutamic acid is most abundant amino acid in a large fraction of all known proteins. Many foods such as tomatoes also contain substantial concentrations of free glutamic acid. It is a "flavour enhancer" so that when small amounts of it are added to food the food tends to taste dramatically better. Soups and many other foods that have been simmered for many hours taste better because a portion of the proteins in the soup components become hydrolysed to free glutamate. It has become common practice to add glutamic acid to foods, but because glutamic acid itself is not especially soluble it is normally added as its monosodium salt. (Less than a gram of S-glutamic acid will dissolve in 100 mL of water at room temperature.) This salt is sold as a flavour enhancer under several different trade names.
Very large doses (more than 3 grams) can produce illness in a few people. This is really a very high dose, but some cooks can go to extremes with the use of this material! Its proper use in the food industry is a good thing, because very small amounts of it can make many foods taste much better without the massive addition of sodium chloride!
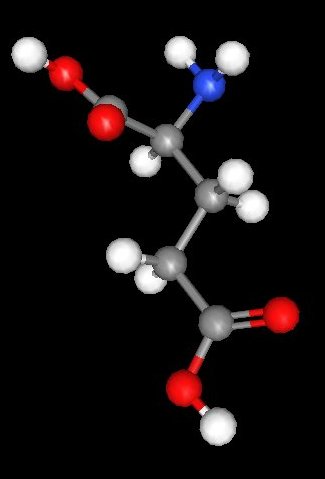
Glutamic acid, like all of the amino acids in proteins except glycine, is a chiral molecule. That means that it can exist as two stereo isomers that are mirror images of one another. The glutamic acid that occurs in ordinary proteins is shown in the image to the right of this text. Because it is chiral it also is "optically active". That means that solutions of glutamic acid and its salts will rotate the plane of polarised light, only chiral materials do this in the liquid phase.
S-glutamic acid, like a large fraction of organic molecules exhibits polymorphism. Polymorphic substances exist in two or more crystallographic forms. The polymorphs of S-glutamic acid are usually designated as the alpha and the beta forms. The beta form is more stable (and hence less soluble in solvents.)
As mentioned earlier free glutamic acid is much less soluble than the monosodium salt. One can take advantage of this fact to produce free glutamic acid from the salt. This can be done by taking a saturated or near saturated solution of the salt and adding a weak acid to it. The best one seems to be simple acetic acid--ordinary household vinegar.
The following procedure is simple and can be performed anywhere without laboratory facilities.
It is best not to use a strong acid like hydrochloric acid, because unless the quantity of acid added is carefully monitored, the strong acid will convert the glutamic acid into its highly soluble cationic form. Acetic acid solutions have just about the correct acidity to cause free glutamic acid to precipitate.
If one allow the solution produced above to stand it will soon be filled with crystals. One way to observe glutamic acid crystals is simply to shake the mixture with crystals to suspend them, and then draw up some of the material with a dropper and put it on a microscope slide and then to place a cover slip on top. Crystals produced in this way will be large and show birefringence.
Both of the polymorphs of S-glutamic acid have orthorhombic crystal structures. The conformation of the molecular chains are different in the two structures.
For the Alpha form a=7.068Å, b=10.277Å, and c=8.755Å.
For the more stable beta form a=5.529Å, b=17.30Å, and c=6.948Å.
An equal mixture of mirror image isomers is referred to as a racemic mixtures. A large portion of pairs of mirror image isomer compounds form crystals that have each unit cell contain equal quantities of each of the isomers. A racemic mixture of the two isomers of glutamic acid form monoclinic crystals. Their crystallographic parameters are as follows: a=5.75Å, b=13.04Å, c=8.43Å. β=103.7 grad.
It is not practical to attempt to obtain crystals of glutamic acid by crystallisation from a melt because the temperature required to melt this material is so high that decomposition occurs.
It is extremely interesting to watch glutamic acid crystallise from solution. Immediately after adding the vinegar to the saturated solution of monosodium glutamate place a drop of this on a slide and cover with a cover slip. The free glutamic acid will soon begin to crystallise. Initially the crystals are very strange, they tend to be elliptical! These are the alpha polymorph. As they grow they begin to develop crystal faces. If one look around on a slide slide made this way one is likely to find needle like crystals as well. These are the beta (more stable) polymorph.
All of the images below were taken using a Nikon S series polarising microscope using a Canon XTi (400D) digital single lens reflux camera and a 2x Olympus photo projection ocular. The image below is taken using a 10X objective with crossed polarisers a few minutes after the acetic acid (vinegar) was added to the saturated monosodium glutamate solution. Both the alpha and beta forms of crystals are present. Most are the unstable alpha form. It forms beautiful "block" crystals whilst the beta form is more needle like.
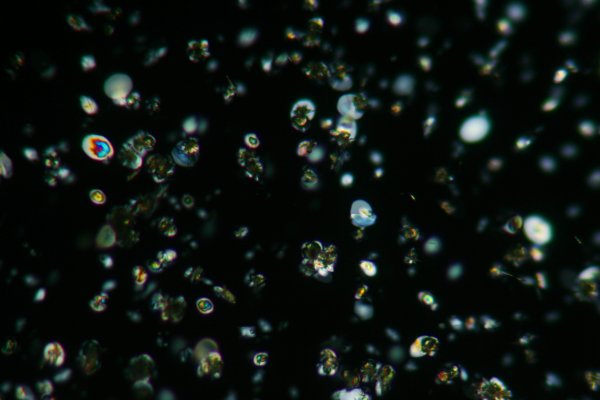
The next image was obtained a few minutes later. It was taken with a wave retarding plate in the optical path and a 10X objective. The beta form generally appears dark, regardless of its orientation relative to the plane of polarisation.
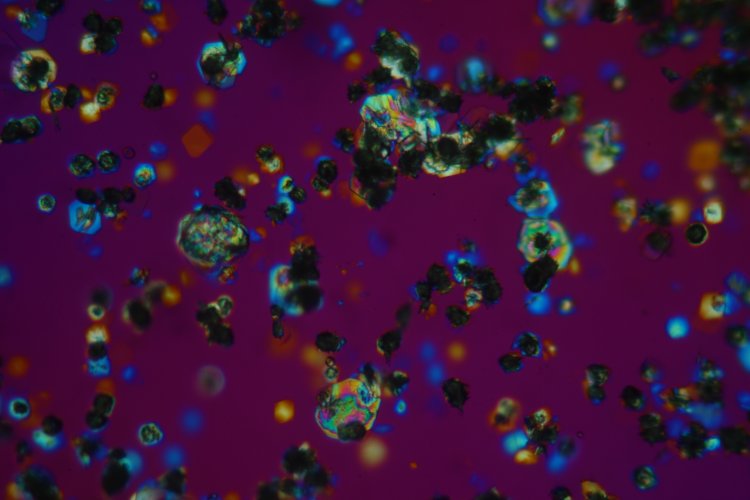
The use of a 25X objective provides a close up of the same area:
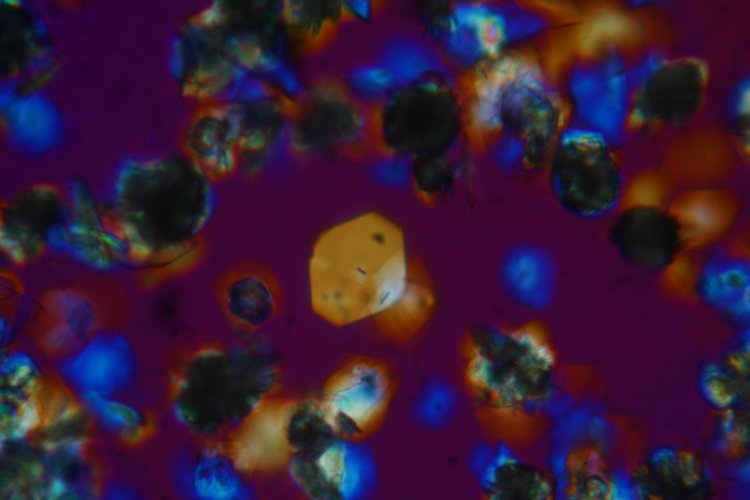
The following image was taken with a different wave retarding plate in the optical path and a 10X objective.
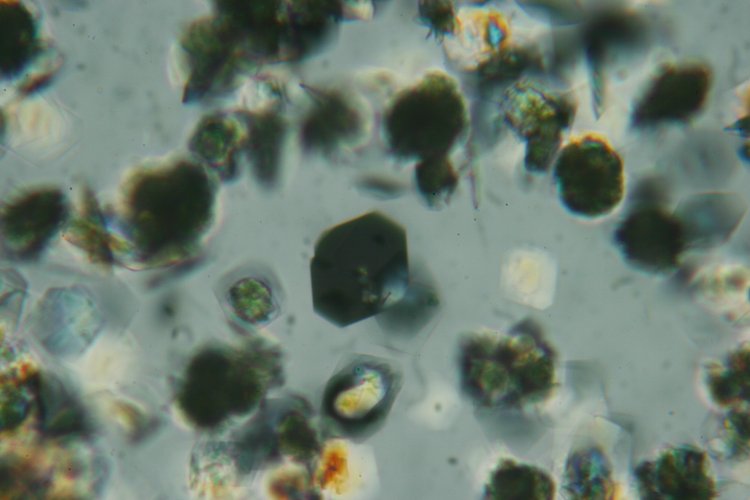
Low power images can be interesting too. The following image was taken using a 2.5 X objective.

The glutamic acid crystals in the next image were formed in a test tube. A drop of the suspension of crystals was simply taken up in a dropper and placed on a slide, and a cover slip added. These crystals tend to be larger, and to have more interesting geometrical shapes. The following image was taken using a 10x objective. Again there both of the crystal types are present.
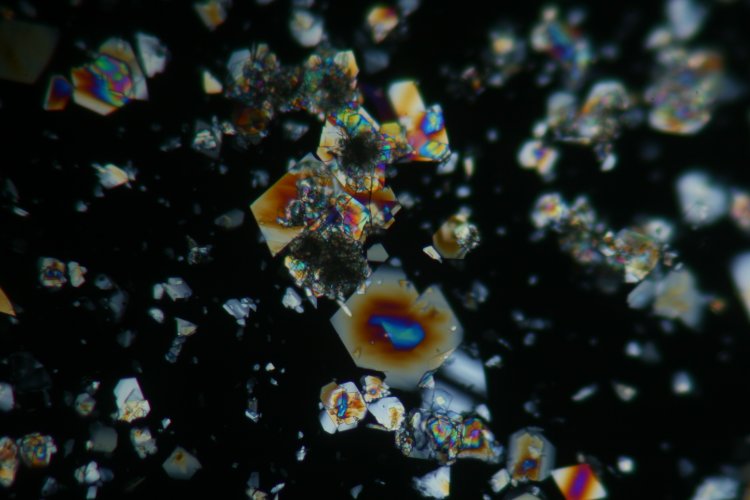
The sample was allowed to sit on the microscope stage for four hours. The next image shows the slide using a 10X objective and the quarter wave plate. (The reason for using this optical setup was simple: the alpha and beta forms are especially easy to identify this way, as mentioned before the beta form tends to appear darker, regardless of the polarisation orientation. Much more of the crystals are now in the beta form. Notice their needle structure, as opposed to the "block like" prismatic alpha form.

The image below shows the same region but with the 25x objective.

There are certainly other ways to react the monosodium glutamate with acid. If one simply add solid monosodium glutamate to acetic acid solutions there is an immediate precipitation of a very fine precipitate of mostly the more stable beta isomer.
All comments to the authors via Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK