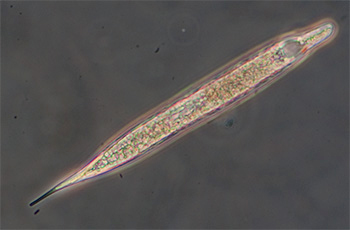
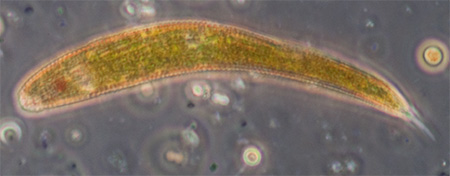
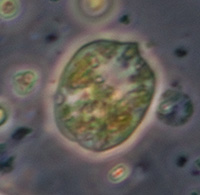
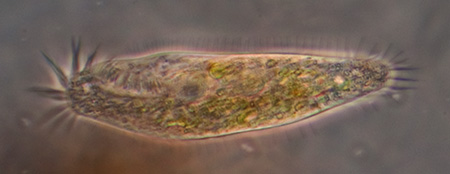
Trials of commercial and homemade microslides (capillary tubes): colonisation by micro-organisms in a back yard pond.
by David Walker, UK
Note added September 2016. The embedded videos hosted on YouTube no longer work. They will be hopefully be restored shortly.
I first came across the potential uses of capillary tubes in Roy Winsby's fascinating article 'Microslide tubes' which was accompanied by the booklet 'VitroTubes techniques and experiments' by Prof. John O Harris (1, 2). I regularly study the fauna and flora in my small garden pond but had never used microslides, so this summer decided to try them.
VitroCom makes VitroTubes™ in a range of lengths and bores that can be mounted on a slide and left in e.g ponds, lakes, streams, soil and designed to mimic the natural capillaries in such habitats. After days, weeks or longer they can be removed to study what life has colonised the tubes. Harris describes in his booklet examples of the tubes' proven uses, e.g collecting organisms from habitats that are otherwise hard to study without disturbing, such as the mud-water interface in aquatic habitats, and they have been shown to attract more unusual types of life such as 'predatory bacterial forms' in soil. He notes that much of the pioneering work on microslide manufacture and their uses was by the Russians (3).
Choice of commercial tubes: The rectangular
form factors were suggested (1, 2) as they can be glued onto a robust support like a normal 3 x 1
inch
microscope slide. The tube size I chose were the
same as those tried by Roy Winsby ie 50 mm long and 2.0 x 0.20 mm ID
which are an intermediate size in the range; as Roy notes, the upper wall thickness of 0.20 mm isn't far off a typical coverslip thickness of 0.17 mm*. Available from C M Scientific and CamLab
in UK. In the tube range the internal height is the same as the flat glass wall depth, other tubes with thinner or greater wall thickness may start to affect image quality with objectives corrected for the typical 0.17 mm coverslip.
*An equivalent coverglass thickness of up to 0.20 mm should be tolerated for dry objectives up to ca. NA0.65 (5). Critical studies with higher perfomance objectives may need a correction collar and/or immersion type.
Homemade mimics? The commercial tubes are becoming expensive at ca. £1 each and sold in a typical 36 piece vial; the price deters buying a vial by a single user or in a range of sizes to experiment with. I split a vial and cost with a colleague, but wouldn't want to break or lose many microslides in a habitat. So in addition to the VitroTubes I also tried a homemade variant using large coverslips glued to a microscope slide with spacers of comparable thickness to the capillary tube ID, and internal width adjusted if desired with spacers; this design is similar to the Russian 'slit peloscope' (Russian workers call the mud-water interface the 'pelogen', 2). A potential advantage of this design is that the more sensitive objectives are presented with the correct coverslip thickness, the upper wall thickness of commercial tubes will vary and immersion studies if desired may potentially be less messy.
In use: The slides I used in the garden pond are shown below with tethers. The supported microslides could be left untethered in more well defined outdoor habitats or in aquatic samples brought indoors; the latter could even use unsupported microslides as Roy Winsby describes (1).
 Right: The two types of microslide tried, shown after 14 days in the pond weed. The upper slide has a 22 mm square coverslip with 0.20 mm actetate strip spacer on two opposing sides, leaving a gap on the other two sides. A second slip on same slide had an internal acetate spacer to
mimic the capillary effect to some extent. The lower slide has two VitroTubes glue to it.
Right: The two types of microslide tried, shown after 14 days in the pond weed. The upper slide has a 22 mm square coverslip with 0.20 mm actetate strip spacer on two opposing sides, leaving a gap on the other two sides. A second slip on same slide had an internal acetate spacer to
mimic the capillary effect to some extent. The lower slide has two VitroTubes glue to it.
To aid recovery, they had a waxed cotton thread (dental floss) tied and taped to them and thread tied to a 35 mm film cassette top with the slide code marked so as knew which slide was being removed.
For well defined outdoor habitats e.g. a local countryside stone water trough, the slide was untethered and just made a note where left for later retrieval.
Tiny spots of superglue transferred via a needle, held the VitroTubes on slide; excess glue may deposit vapours on glass. The VitroTubes were easily nudged free after water immersion, which proved useful because they could be wiped clean then placed on a clean slide for study under the microscope.
If the tubes are left on the slides they were mounted on for microscopy studies, the water between the tube and glass slide was hard to dry and could leave additional deposits to detract from studies.
Superglue was also used for the coverslip design of microslide with acetate film spacer 0.20 mm thick, but these proved too delicate and slips came off too readily when handling and cleaning. The excess glue also left white deposits on the glass in the capillary area. A better glue is needed, thin enough not to affect the spacing desired, resistant to water immersion and one that doesn't leave vapour deposits nor atttacks the spacers if plastic. A trial with clear nail varnish was unsuccessful.
The VitroTubes lasted for some hours with little drying back of the capillary sample. Much more care was required with the homemade variants which dried back rapidly, as would a normal temporary aqueous mount with coverslip.
The exposed uncovered glass area on a slide, or just blank slides, could in principle be used for monitoring what became attached over time, so-called 'fouling slides' (2), but over the weeks I tried the slides, no marked colonisation was seen on the exposed glass in any of the microhabitats tried.
|
Right: the small butyl-rubber lined back garden pond built by my brother Ian 16 years ago. It's about 2m long, 1m wide and 1m deep at deepest point. The garden is small, ca. 7 x 5m. The 35 mm film caps on pond side and tied to wax string leading to slides in three habitats are just visible and highlighted with red lines. Two slides were left in each habitat for initial trials to allow removal at different periods for comparison. The farthest end of pond is gently sloping with little mud and where one slide group was left. It has a natural population of frogs; very weedy typically with Canadian pondweed in summer. Larger life includes freshwater shrimp and smaller water beetles. The bottom mud is light, deep and hard to tell by feel where a slide has been placed in it. |
|
Time to leave in habitat? This is one of the parameters that could be studied. For my own first trials I took note of Roy Winsby's observations that he found relatively little life after 3 days but did after a further 10 for a pond sample brought indoors (1), so the slides were left for a minimum of 12 days.
Pond trials: Supported tubes were left in three microhabitats: 1) laying flat on the sloped plastic lining of the pond in a very lightly muddy area, 2) flat and supported within the dense weeds near the surface and 3) inserted vertically at or near the mud / water interface in the mud in deepest part of the pond (the orientation suggested in the literature (2)).
Organisms found. A gallery of stills and video clips of a selection are shown below with notes on both the creatures found to date and first impressions on microslide use. All images / film taken with a parfocal Nikon D5000 digital SLR on a Zeiss Photomicroscope III, with Zeiss 10x Kpl W eyepiece
on short collar for projection. Most useful objectives were Zeiss phase 10/0.22 for scanning, and Neofluar phase 16/0.40, 25/0.60 and 45/0.75 for more detailed studies. Electronic flash was used for stills, using a half silvered mirror as beam splitter sat on field iris with flash gun laid on base (see this article for set-up).
The Nikon D5000 DSLR is capable of high definition 1280 x 720 (1080i) 24 fps video capture, but early trials suggested this was overkill and rapidly 'ate' memory cards. The 640 x 424 (720p) 24 fps mode proved more practical. The ability of a modern budget DSLR to seamlessly capture either stills and/or video was an extremely valuable feature and the first time I've used the video mode in earnest. The downward facing tilting LCD Live View screen
of the camera allowed stills/video to be monitored in real time while still sitting at the microscope.
My skills at identifying the tinier freshwater micro-organisms to genera or species is limited and relies on closest match of critter to picture using books like Pennak (4), so any firmer ideas on the IDs are welcomed.
The bottom and mud samples
These were both initially studied after 12 days. The vertical tubes in mud had less visible life, they may have been placed too deep as hard to gauge by feel how they were placed in the soft mud. A second vertical slide with two VitroTubes on was removed from the mud/water interface after four weeks and again little life was seen compared with those left in the weed or on flat plastic bottom of pond liner. Bringing mud samples indoors as suggested (1, 2) would probably be a more rigorous way of studying the mud-water interface.
The microslides placed on plastic bottom proved the more interesting and a selection of organisms found shown below. As remarked above, the coverslip design with 0.20 mm acetate film and superglue proved too delicate to study, but those that survived didn't seem to have the range of life of the VitroTubes, but of course inconclusive with such a small set for comparison.
Bacteria and algae
|
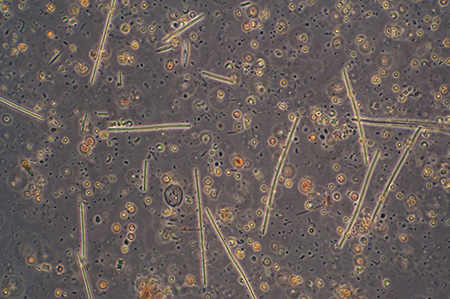
General view with 16x phase objective, showing the high density of smaller microorganisms and fine detritus. Field of view 512 um. |
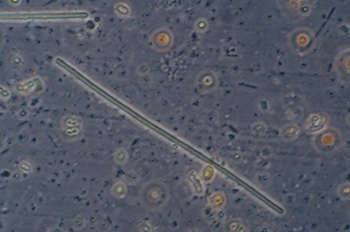
These almost featureless, but segmented in some views, presumably bacterial forms, were plentiful in the sample. 40x objective, phase contrast, length 114 um. |
|
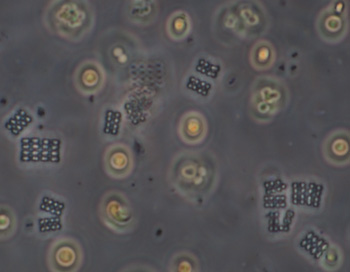
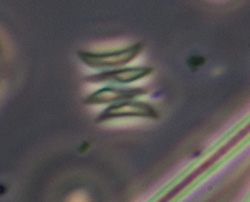
Crop of field with 40x objective. The rectangular colonies may be Merismopedia or similar; individual elliptical cells ca. 2.5 um long. The spherical forms, ca. 8 um in diameter, possibly the algae Chlorella or similar, were abundant at the time of sampling as also present in open water, giving the water a pale green colour. |
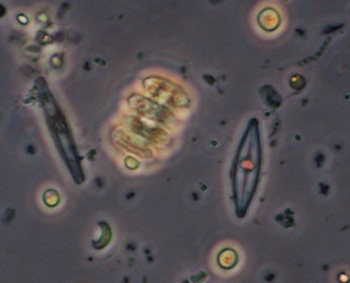
Crop of field with 40x phase objective, field of view 90 um. Two species of small diatom, crescent shaped algal? forms (too small for a desmid?), and a tiny bacteria form top right scan be seen. |
|
Phase 25x objective, colony height 17 um, possibly the algae Scenedesmus or similar. |
. A bacterial form, 40x phase objective, length 60 um. Possibly Anabaena or similar. |
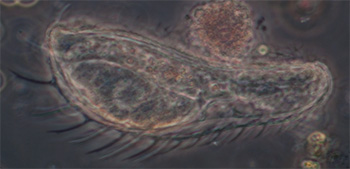
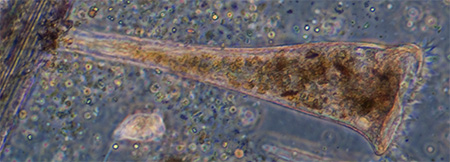
Protozoa
|
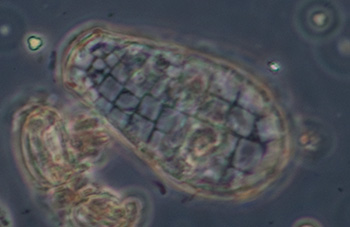
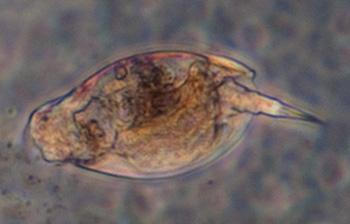
Testate amoeba, possibly Difflugia, case length 185 um, Zeiss 10x NA0.22 phase objective. See video below. |
Another Amoeboid protozoan, genera uncertain, 'body' length 50 um. Has thin 'growths', possibly reticulopodia, unlike the thicker pseupopodia of some Amoeboids. One longer than others and thickened at base extending to the left. |
|
Case of a testate amoeba, length 45 um, 25x phase objective, with distinct rectangular plates, possibly Quadrulella. From pipetting the mud / water interface from an open mud sample kept indoors, rather than capillary tube. |
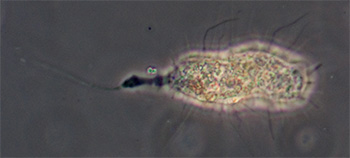
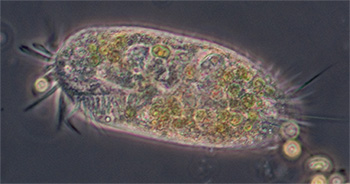
Ciliated protozoan, possibly Uroleptus or similar. Phase 16x objective, length 248 um. The largest protozoan found in the mud capillary tubes to date and common. See video below. |
|
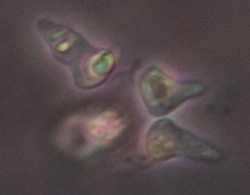
Possibly a Euglenoid protozoan, length 95 um, rigid body with red eyespot and forward vacuole but no obvious flagella or the strong green colour. The cell contents and / or body seemed to be in continuous spiral movement, first one way then the other in a rhythmical fashion. See video below. |
Possibly another Euglenoid protozoan with red eye spot, likely Euglena, but very mobile and twisting body motion. Phase 40x objective, length 80 um. |
|
Phase 40x objective, length ca. 23 um. |
Ciliated protozoan, 16x phase objective, length 130 um. See videos below which compare phase and DIC imagery. |
To compare with the outdoor capillary tube experiments in the pond mud, a mud sample has been brought indoors ca 1 cm deep in a jam jar of deep pond water and pipettted samples from interface taken at intervals. Spotting tiny organisms by stereo microscope first was difficult. For random samples directly studied on the compound microscope, it maybe pot luck as to what is found. An inverted microscope with long working distance objectives (not posessed by me) maybe better for these sort of studies.
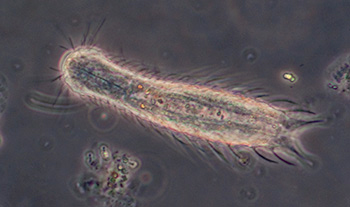
Multi-cellular organisms
|
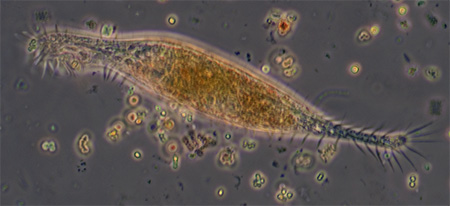
Gastrotrich, a fleeting glimpse, probably Chaetonotus, length 138 um, Zeiss Neofluar 25x NA0.6 phase objective. About three were seen in a tube with fine detritus. The internal height of the tube used is 200 um and it proved difficult to track and study faster moving creatures such as these in such a large water column. With a traditional temporary water mount with coverslip
on slide, various methods at controlling movement can be used. |
A gastrotrich, probably Chaetonotus genus, presented in an unusual way which initially made wonder if a protozoan, but thank you to Walter Dioni who pointed out the embryo and its correct attribution. Length 107 um, 40x phase objective.
|
Rotifers: no bdelloid rotifers were seen to date; a small fast moving species (flattened with shell) was seen but near impossible to study or photograph because of the water column size and rapid tumbling motion.
I tried flushing out the tube with a minimum of water, searching the water with a stereo for recovery of the rotifers to study under a normal slide / slip mount, but was unsuccessful to date.
Videos from VitroTubes left on bottom in light mud area. (hosted on YouTube)
(The video still created by YouTube gives a rather corrupt view of the video quality.)
Protozoan, DIC. The optical sectioning of DIC shows the cilia on upper surface. The comparable phase study does not show this feature well. The DIC imagery didn't seem as crisp with the VitroTubes as normally seen. This technique maybe more sensitive to the none standard viewing conditions.
Same specimen using phase contrast. Phase does highlight edge-on cilia and cirri well but not the upper surface features which DIC shows well. Phase seemed more tolerant to the VitroTubes although the halo effect perhaps more pronounced.
Ciliated protozoan, possibly Uroleptus or similar.
Gastrotrich, phase contrast, on a rare occasion that it wasn't in 'turbocharged' mode and moving along the corner of the VitroTube.
Protozoan, possibly Euglenoid type. Shows the rotational movement of the body and/or body contents. In clockwise motion only the cell contents seem to rotate as eyespot didn't, in anti-clockwise the eyespot rotates as well.
The testate amoeba Difflugia extending a pseudopod searching for food. Objective 16x phase.
Weed samples
Microslides were placed in the Elodea weed but was uncertain whether microorganisms would favour a sterile 2 x 0.20 mm glass capillary cf the presumably more appealing food source of weed surfaces / open water. But the VitroTubes removed after 14 days and studied with the 10x objective showed a seething mass of microlife at a density I more normally associate with forced cultures such as hay infusions.
General field of capillary tube after 14 days in weedy area of pond. Density of microlife far higher than open water sample freshly taken in same area.
For comparison I also looked at an open water sample from weed area in case I'd been sampling the pond plankton at a particularly 'pea soup' time of year. The density of microlife was far lower, with just an occasional organism seen swimming across the same field of view. I'm not entirely sure what roles the VitroTube plays to have such a marked effect at concentrating the microlife from open water. I don't believe there is a capillary action when a tube is totally immersed, but perhaps the long tube acts as in part as a trap and organisms that enter cannot readily get out rather than preferential colonisation; comments welcomed!
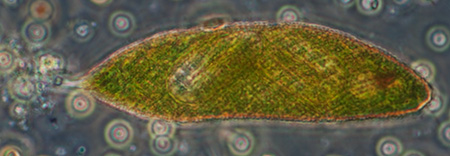
Typical microlife found in capillary tubes left amongst weed - protozoa and multicellular organisms
|
Stentor, phase contrast, length 390 um, 10x phase objective, attached to the acetate spacer of the 0.20 mm spaced coverslip. |
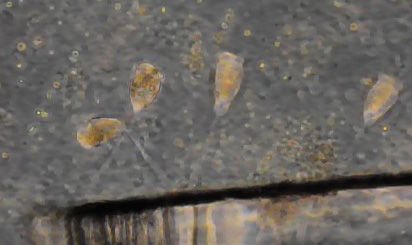
Protozoan, 40x phase objective, length 105 um, possibly Euglenoid, likely Euglena, a number of these were seen in the coverslip capillary slide. Distinct cross hatching texture on surface. Maybe the same species seen in the mud in a more contracted state. |
|
A small crop of image capture to show the tiny organisms that were abundant in the sample. Phase contrast, 40x objective, length of each ca. 11 um. Not certain what type of algae / protozoa this is. |
A ciliated protozoan, 25x phase objective, length 115 um. |
|
Vorticella, like Stentor also favoured the acetate spacers as support in the homemade capillaries left in the weed. Phase 10x objective. |
Rotifers were more abundant in the VitroTubes or homemade capillaries left in the weed cf the mud. But difficult to study or photograph because of their speed in the deep, fixed water column. Phase 10x objective, small crop of field, length with 'tail' 121 um. |
Videos from weed samples
Rotifer browsing through the densely populated water column, 40x phase objective.
Protozoan, 40x phase objective.
Vorticella, 40x phase objective. They seemed to favour the homemade wide slit capillaries to the narrow bore VitroTubes.
Stentor attached to acetate spacer in homemade capillary, 10x phase objective.
Comments on using microslides to date: I've greatly enjoyed trying this method of collecting micro-organisms and has added structure to my more typical 'pot luck' sampling of plankton and weed at various times of the year and also prompted my first real study of the pond mud.
From an admittedly limited set of studies so far ....
Pros:
- eager sense of anticipation at what might be found after waiting a week or more!
- linear scanning of a tube minimises overlooking any organisms present; without the usual x-y scan required of large coverslip areas and potential missing of organisms
- to date has collected smaller microorganisms potentially overlooked by other methods, e.g. amoeba, other protozoa and gastrotrichs
- tube can be left in a Petri dish of water and restudied a day or more later as many organisms remain in the tube
- allows a study of well defined microhabitats
- a variety of parameters invite study, e.g. time of year, microhabitat of pond, immersion time, orientation in mud etc.
Cons: (of the 50 x 2 x 0.20 mm ID tubes tried)
- the 200 um high water column can make study of fast moving organisms difficult; capturing stills and/or video aided further study in detail to identify
- organisms that move into the tube edges are hard to view, and preferred habitat of some
- becoming expensive to buy e.g. in a range of sizes and may deter use by individual enthusiasts or in educational establishments where easily broken or lost, but cheaper homemade variants with coverslips, spacers and suitable glue could be tried
- the lower and upper internal walls can become populated with bacteria preventing highest quality imaging of organisms in the water column.
- critical studies using VitroTubes with higher perfomance objectives may need a correction collar and/or immersion type; homemade variants using coverslips may be a better route for such studies
- the VitroTubes usually contain fine detritus after leaving in a pond, to date this has been found hard to clean out for tube reuse. Ultrasonics maybe successful but not tried.
The potential value of my own homemade variants of microslide is inconclusive at present as the glue used wasn't good enough for extended immersion. For those slides that did survive, those placed in the weedy area certainly attracted a high density of microlife, especially the larger organisms such as rotifers, Stentor and Vorticella which were not seen in the VitroTubes. If the VitroTube's long length cf their cross section has a trapping action of microlife to some extent, homemade variants that more closely mimicked this form factor could be tried, e.g. by butting large coverslips side by side with long internal spacers.
Comments to the author David Walker are welcomed.
Acknowledgements. Thank you to Roy Winsby for drawing attention to the potential use of microslide tubes in his 1992 article and for the accompanying reprinted booklet by Prof. Harris. Thank you also to Walter Dioni for useful discussions on the technique in general, suggestions for designs of better homemade capillary tubes and useful comments on likely identification of some organisms seen. Any errors are solely mine.
References and further reading
1. Roy Winsby, 'Microslide tubes', 'Micro Miscellanea', 'Newsletter of Manchester
Microscopical and Natural History Society'. May 1992, Issue No. 22, pp. 1-2.
2. 'VitroTubes techniques and experiments' by Prof. John O Harris. Link is to free download in Acrobat format from the VitroCom website.
3. B V Perfil'ev and D G Gabe, 'Capillary methods of investigating
micro-organisms', Oliver and Boyd, Edinburgh, English translation by
J M Shewan, 1969.
Unlike many textbooks, this 600+ page book currently
sells for about $15 upwards on www.abe.com.
The first three chapters which includes 'Principles and methods of capillary microscopy' and 'Studies of Microlandscapes' are likely of most value to enthusiasts, later chapters deal with more complex techniques, equipment and tube manufacture.
4. R W Pennak 'Fresh-water Invertebrates of the United States', 3rd edition, Wiley, 1989.
5. Zeiss 'Optical Systems for the Microscope', page 16, a 43 Mbyte download in Acrobat® format from Gordon Couger's science-info.net website.
Microscopy UK Front Page
Micscape Magazine
Article Library
© Microscopy UK or their contributors.
Published in the September 2011 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk.