PVA-L.-
Polyvinyl alcohol-Lactic acid medium
Lactic
acid is not a forbidden substance. It is a clear liquid which has a mild
odor and is pleasing to work with. As we saw when I presented lactoglycerol
it is considered a powerful clearing agent coming only third to chloral
hydrate and phenol. It is also an expensive product of very extensive industrial
use, and more or less difficult to get in small quantities. But try old
drugstores and suppliers to school laboratories, surely you can get the
quantities you need at a reasonable price, as I did.
Probably
the first PVA-lactic acid mounting media was the one published by Omar
et.
al. in 1978. You can prepare your own PVA-lactic mountant, with an
RI of approx. 1.39. One professional formula I obtained from several sites
on the Web is this:
PVA
.....16.6
g
Water
....100
ml
Lactic
acid
..100 ml
Glycerin
.
5 ml
Dissolve
PVA in water, add the lactic acid while mixing vigorously. Add the glycerin
and leave for 24 hours before use.
My
(amateur style) is of this formulation:
OGlue
(See Part 2)
.
.30 ml
Lactic
acid
...15 ml
glycerin
1.5
ml
It
has a very good consistency. PVA-L is a universal mountant. Suitable subjects
include small arthropods, parts of the same, microfungi, some algae, some
botanical preparations. You can transfer the subject directly from water,
alcohol or glycerol to the PVA-lactic media, or if the objects are really
dark, you can use a preliminary clearing bath (2 parts lactic acid:1 part
glycerin), then transfer your subjects to glycerin. Dilute with water if
it is convenient. Another clearing medium could be simply lactic acid at
a 1% or 5% strength. Experiment to find the best medium and the time your
material needs to be cleared. Some subjects clear in minutes, others require
as long as several days. Mount as above.
My
lactic formula dries very rapidly. In some hours the media will set.
As you will see the margins harden enough to clean up as I described for
the CPG (commercial PVA glue) medium and seal the preparation with a double
layer of nail polish. Some references state that even so sealed the PVA
formulas can evaporate solvent, dry out and after some months peel off
the glass. I suspect that NPM (nail polish mountant) can have the same
behavior.
It
is recommended to seal with a really hard sealant. In the USA the Red
Glyptal, a varnish to protect electric machinery
(also used by palaeontologists to protect fossil bones) is recommended.
I have still not found a substitute in Durango, and must use some automotive
paints, hoping this helps.

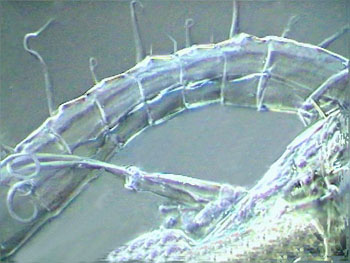
PVA-L, mosquito larva head ventral
|

PVAL, mosquito larva buccal armature
|

PVA-L, mosquito larva pecten airtube
|
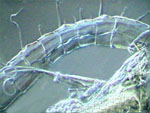
PVA-L, female Diaptomus antenna
|
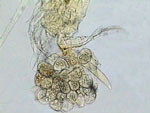
PVA-L, Diaptomus ovisac
|
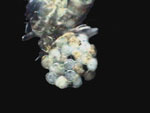
PVA-L, Diaptomus ovisac (2)
|
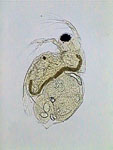
PVA-L, daphnia
|
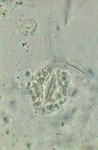
PVA-L, epithelial cell of leaf underside
|

PVA-L, fly wing-1
|

PVA-L, fly wing-2
|
A
few comments about 3 of the above pictures. The diaptomid first antenna
is portrayed in my domestic version of the scanning microscope; (Rheinberg
plus a vertically displaced central stop somewhat out of center). The copepods
were washed from the lactocupric fixative with water and mounted directly
to the PVA-L. In spite of this, the eggs don't show the collapse that they
suffer with FG and FGL. The ovisac in darkfield was photographed using
the same method as for the antenna, but with the central dark stop well
centered and adjusted, and I use a Rheinberg filter of another color. (Well...
as the black field doesn't register so black as I'd like it in the
original picture, I made a sustitution with the aid of PhotoPaint.)
Commentaries.-
Contrary to the CPG, of a lower RI, this lactic formula I prepared has
a similar behavior to NPM (nail polish mountant) and gives similar results
in the short time. With more time, (one or two weeks) it almost clears
the internal tissues and gives a neat view of the chitinized structures.
In several weeks the extent of clearing makes it more difficult to discern
the thinner structures like spines and setae. You can try to stain the
chitinous skeletons by treating them, before mounting, with lactic
acid colored with a few drops of methylene blue.
(I picked up this trick from the web, but I have not tried it until now.)
Or as a last resort use good oblique illumination or phase contrast.
So
you must select very well the materials you mount in this kind of mountant.
Or try the solution used by Robert Constantine
for his Damar mountant. See also my commentaries to the Glycerinated
Lactic Gum (to be published in Part 3d).
The
literature on PVA mountants has many enthusiastic appraisals and also some
totally dismissing opinions. The late G. Ramazzotti,
in his monograph about the Tardigrada says that many times (as I have experienced
with the OGlue) the polyvinyl-lactophenol formula he used developed voids
under the coverslide, without any known reason. He also states that on
the contrary, he had preparations that lasted many years without faults.
I
think that if one can obtain a PVA of the recommended density (24 32
centipoises), the professional formula is a safe mountant, easy to mix,
that merits more additional experimentation. But if you are unable to get
it, browse through the art or the office supply houses, as I did. Try the
PVA paper glues, they deserve a try.
Some
time ago I started to question why the PVA was restricted by professional
microscopists to the lactophenol formulas (now additionally restricted
to lactoglycerol formulas). So I tried with relative success the CPG adventure
(see part 2). Now I propose you indulge in the heresy and design a PVA
based media of mild clearing action, a lot less acidic, that could be of
a more general application (including those little tardigrada, with his
delicate calcified pieces). My own experimental version is below which
I've put on the trial to monitor its behavior for the next few months.
PVA-G.-
Polyvinyl Alcohol-Glycerol medium
OGlue
10
ml
Borax
Water*..
.
4 ml
Glycerol
6
ml
*Saturate
water with granulated borax (>6 g of borax /100 ml water). Use the supernatant
liquor.
Editor's note added Nov 2004. The preparation method
of PVA-G is quite critical as apparently the above ingredients
can also make synthetic 'slime'. See Howard
Webb's Nov. 2004 article .

PVA-G, fly wing-1
|

PVA-G, fly wing 2
|
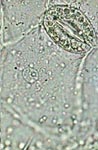
PVA-G, epithelial cell of leaf underside
|

PVA-G, mosquito larva head
|

PVA-G, mosquito larva pecten airtube
|

PVA-G, mosquito larva pecten (1000x)
|
Comments
to the author, Walter
Dioni, are welcomed.