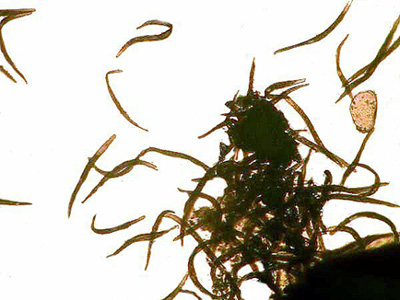
|
Copper Salts, Ciliates, and A Taxonomic Puzzle by Richard L. Howey, Wyoming, USA |
A couple of years ago, I was experimenting with making up some slides of crystals of copper salts some of which are quite beautiful and they will form different patterns at different temperatures. I happened to recall that copper salts, especially copper sulfate, were sometimes used in lakes and reservoirs to control algal blooms. Since I had the solutions right at hand and since I had some good cultures of ciliates going, I decided to try out some solutions to see what results I might obtain. I was particularly interested in testing the effects on contractile ciliates and some of the hyperactive ciliates. The frustration involved in trying to study these two types of critters in detail can produce copious quantities of profanity which, if written down and published, might well rival the Congressional Record in sheer volume. Ciliates are complex beings and when they zip through the water like some mad Floridian on a water jet ski, they are virtually impossible to examine without taking drastic measures. On the other hand, organisms which, just when you think you have them in the right position such that they will reveal their inner secrets, contract before your very eyes, are equally maddening.
Walter Dioni has provided two valuable articles in Micscape [in July 2002 and October 2002] on using a highly dilute solution of formaldehyde as a relaxant for certain micro-organisms. He cautions that this solution is generally not suitable for protozoans, although, apparently, he has had some success with Vorticella. He also points out that different organisms may require different concentrations of the formaldehyde solution to be effective. This is an important lesson to remember in the application of any sort of relaxant, narcotic, or anesthetic, killing, or staining solution. Turtox used to publish a little brochure on anesthetizing agents for aquatic invertebrates with a very helpful table of organisms and appropriate reagents. Unfortunately, I can’t reproduce it here, because I suspect it’s still under copyright. However, if you can find a copy of it in a library, you can certainly xerox it for your own use. It was copyrighted in 1959 and published as “TURTOX SERVICE LEAFLET No. 2" and titled “PRESERVING ZOOLOGICAL SPECIMENS: NARCOTIZATION, FIXATION AND PRESERVATION”.
For ciliates, the substances I have found most effective have, until recently, been nickel sulfate and potassium iodide. My preliminary experiments with copper salts, especially copper acetate, have convinced me that these reagents are well worth further systematic investigation. In poking around in the older literature, I discovered a formula for a copper acetate fixative that is described as providing a “delicate” fixation. Thus far, I have been less happy with the fixative than with a 2% solution of copper acetate and some modifications, which I will discuss later. Thus far, I have had good results with Spirostomum, Stentor, Vorticella, Euplotes, and Oxytricha. Lacrymaria, however, did not fare so well, so I will have to try adjusting the concentration. (Hope springs eternal.) In some ways, it is the ultimate test due to the extreme extensility of its “neck”—up to 10 times its body length! [See my April 2000 Micscape article on Lacrymaria.]
When working with contractive and/or highly reactive organisms, the number of variables can be daunting. Consider some of the potential problems and options:
1) Should the reagent be administered to a few organisms at a time on a slide or to a larger number in a small vial? The former has the advantage of allowing you to observe the effects immediately; the latter has the advantage of immediately mixing more thoroughly and increasing the probability of obtaining a larger number of organisms in the desired state.
2) If you decide on the slide option, should you add a drop of the reagent directly to the drop of culture to try to get as immediate and uniform an effect as possible? Or should you place a cover glass on the drop of culture and then place a drop of the reagent at one end (or at both ends) and let capillary action gradually introduce the reagent? A drop of culture on a slide is a surprisingly complex environment. The relative cleanliness of the slide can effect whether or not you get a nice well-behaved bead of water, a sort of shallow miniature dome, or whether the drop spreads out thinly and randomly and exasperatingly over the whole center of the slide. However, even if you get a well-formed drop, there may still be difficulties. There can be a biophysical disaster when certain organisms encounter the surface of a water drop. For example, I have many times seen Lacrymaria swim to the edge or to the top of a water drop and simply explode; it is as though they had been vaporized. Moreover, when we add reagents to the drop, we may very well alter some biophysical properties and increase the difficulties that some organisms have in coping with surface boundaries.
When we add a cover glass to the drop, we are also making significant changes in the micro-environment, especially with regard to pressure gradients.
3) If you use tubes, a small amount of culture can be introduced and then the organisms quickly flooded with the reagent, thus mixing the reagent with the organisms rapidly, but creating a large scale micro-turbulence and increasing the probability that highly contractile and extensile organisms, such as, Spirostomum, Stentor, and Lacrymaria will end up badly distorted.
4) Should the reagent be used at room temperature or warm or hot, or, for that matter, chilled? Relatively little work has been done employing chilled reagents and yet, such a technique might prove effective with organisms which typically live in very cold water such as the alpine lakes in the mountains here in Wyoming. Sometimes very simple techniques or simple modifications of more complicated techniques can lead to significantly improved results. For example, some kinds of rotifers can be killed in an expanded state by simply flooding them quickly with boiling water. [CAUTION: Never heat a reagent, unless you know exactly what the effects will be and unless you have taken appropriate safety precautions. Some reagents when heated produce poisonous fumes, others may produce flammable or explosive gases, and still others may splatter producing serious chemical burns. Such chemicals are particularly dangerous if they happen to get into the eyes or lungs; so, this is NOT an area to experiment with randomly!]
So, which are the best procedures? Obviously, the ones which work best for you and I’m not being flippant. The history of microtechnique is filled with interesting cases of investigators who publish a procedure with which they have gotten excellent results and then someone else comes along and says that the procedure is useless and if you want really good results, we should try the second guy’s technique. Staining techniques are a good example; just think about how many types of hematoxylin staining techniques have been developed including a series of different recipes for the staining solution, include those of Mayer, Harris, Ehrlich, Heidenhaim, and Delafield, just to mention the major ones.
Thus far, I have mostly used the 2 slide techniques. If, on a particular day, one method gives good results, I’ll stick with that. If, however, the results are only mediocre, I’ll try the other method. With a number of reagents and stains, the most desirable effects are achieved only after a period of some minutes or even hours. This means that a slide preparation can dry up, thus ruining your experiment. To counteract this, I added some glycerine to my copper acetate solution. The amount is a bit tricky, because you want enough so that as the water evaporates from under the cover glass, there will be enough glycerine to keep the specimens moist and minimally distorted. On the other hand, if there is too much glycerine, it will distort your specimens since it is hygroscopic, that is, it absorbs water and in too great a concentration can quickly remove water from your specimens, radically distorting them and destroying your preparation. Start with 1 ml. of glycerine to 30 ml. of copper acetate. If that’s too strong, add more copper acetate solution to your reagent bottle. If it’s too weak, try adding another 1 ml. of glycerine. Repeat until you decide to give up microscopy and take up stamp collecting or until you get satisfactory results. Even though this is a temporary mount, I have had some preparations done with stains and the addition of glycerine, which were still useful even after several months.
While we’re on the subject of staining, let me mention a staining procedure that I have used in conjunction with copper acetate. My sister and her husband, who kindly tolerate my eccentricities, went lake fishing in Minnesota last summer and so I asked my sister to send me a few water samples and, sure enough, a small box arrived with 5 little jars containing just enough organic accompaniment to make them interesting, but not enough to make them foul. In one of them she had placed a few shreds of tree bark which had been in the lake along the shore. At first, there was nothing of note in the sample, so I placed it in a small culture dish, added a grain of rice and let it sit for a week. After a week, big, lovely specimens of Spirostomum appeared and cultured readily.
However, even after several months of examining it off and on, I am still uncertain of its species. It is a large one, over 1000 microns, of approximately the same width from the posterior to the anterior, has a large elongated posterior contractile vacuole, and a central cytostome. At first glance, I thought it might be S. minus, but that species tends to be somewhat thinner and also tends to taper a bit at each end.
Then I noticed a central ovoid structure near the cytostome and I thought—aha!, S. teres, which has a single ovoid macronucleus. But this organism is rather too large for S. teres which is one of the smaller species of the genus. It didn’t seem quite big enough for S. ambiguum, although it does fit into the size range of that species, but typically S. ambiguum will, in culture, produce some very large individuals and that has not been the case in these cultures. Furthermore, S. minus and S. ambiguum have chain micronuclei which consist of bead-like structures and can extend from 1/3 to over ½ the length of the organism. However, using just traditional optical contrast techniques didn’t give me a glimpse of any such arrangement—just the centrally located ovoid structure. So the next step was, of course, to try a nuclear stain and hope that the distortion by the violent contractions of Spirostomum wouldn’t totally obscure the information which I wanted regarding the macronucleus. One of the nuclear stains which I like best is Methyl Green Acetic. Use a concentrated solution of Methyl Green acidulated with acetic acid to about 1%. Use live organisms, otherwise the stain is not really effective. In working with the Spirostomum (which may be S. intermedium), I introduced a couple of modifications. First I added a bit of glycerine to the stain and secondly I tried using the stain both separately and after treating the Spirostomum with copper acetate. I know I said that one should use living organisms and the interesting thing is that the copper acetate first seems to block ciliary action without killing the organism, then after some seconds the organisms may begin to undergo spasms of contraction. So, sometimes, one is able to follow up quickly with Methyl Green Acetic and get very nice results. I have been using the slide-coverglass-capillary method. The results seem to depend strongly on the concentration of the solution and the timing in administering them. Sometimes, the organisms end up radically distorted, whereas, on other occasions, they remain reasonably well-extended. However, even distorted specimens may provide the information one needs.
Remember, the immediate issue here is the nature of the nucleus of this particular species of Spirostomum. As it turns out this species does indeed have a moniliform or “beaded-chain” macronucleus. However, this nucleus seems a bit different from that of S. ambiguum or S. minus in that some of the “beads” are elongated and appear more like miniature ’sausages’ than “beads.” Perhaps this is a result of the reagents but, in using the same techniques with S. minus and S. ambiguum, I have not noticed such elongated nuclear elements. The next step was to use a fluorochrome (fluorescent stain) which is a powerful nuclear stain, namely, Acridine Orange. This technique confirmed that the nucleus is indeed moniliform and possesses small “beads”, longer “sausage-shaped” parts and very thin “threads” connecting the various parts which comprise the entire nucleus.
So, which species is it? I turned to the old classic reference, Kudo’s Protozoology. The 3 viable candidates are described as follows:
S. ambiguum—1000-3000 microns, beaded-chain macronucleus, many micronuclei, peristome—2/3 of body length.
S. minus—550-880 microns, beaded-chain macronucleus, no mention of micronuclei or peristome.
S. intermedium—400-600 microns, bead-chain macronucleus, no mention of micronuclei or peristome.
By using copper acetate, I was able to examine my species, before lysis occurred sufficiently to hazard the conjecture that the peristome runs about ½ the body length.
Thus, still being confused, I turned to the recent electronic available information on the Japanese website: The Protist Information Server, here’s what I found:
S. ambiguum—macronucleus, moniliform (beaded-chain), peristome 2/3 body length no mention of micronuclei and the size range differs considerably from Kudo—525 to 1200 microns!
S. minus—Here it is stated that the macronucleus is ellipsoidal! This, I think, is simply wrong. All of the specimens of S. minus I have examined had moniliform macronuclei.
S. intermedium—macronucleus moniliform, 300 microns, peristome ½ body length.
All of this raises the issue of the degree to which we can rely on gross morphology to make species differentiations and this is by no means a new problem. Perhaps the difficulty could be solved by genetic sequencing, but even among professional biologists, few will find that worth the effort and expense. One might even be tempted to ask: What difference does it make? With some groups of micro-organisms, bacteria, for example, differentiating species within a genus can be of enormous importance, since within the same genus, one sometimes finds organisms that are beneficial, benign, or pathogenic.
All three of these species of Spirostomum have not only the virtue of being harmless, but of being wonderful to watch as well; I regard them as the great micro-whales of pond protists.
My experiments with copper acetate are in their infancy, but the preliminary results are encouraging. In part this is a consequence of the fact that at the “proper” dilution, this reagent will cause Spirostomum to swim in a coiling and entwining fashion, then to gradually slow their movement as they extend to full length, then to cause them to essentially stop moving, although some cilia are still active and , finally, to begin contractions. These contractions are, in some specimens, violent and convulsive, while in others, they are rather mild, producing relatively little distortion. In any case, the process lasts long enough to obtain some good images either through videography or digital photomicrography.
IMAGES
Spirostomum presents many difficulties, but one of its virtues is that it is rather readily cultured and thrives on benign neglect, within reason. A culture that many other organisms find foul is often quite suitable for Spirostomum. A few organisms, a small culture dish, a few boiled wheat grains and, in a matter of a week or two you will likely have abundant Spirostomum.

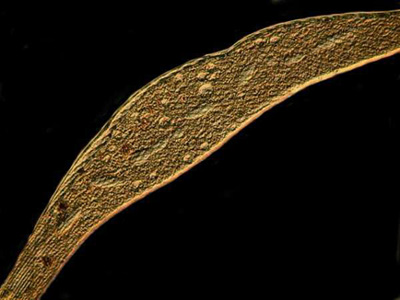
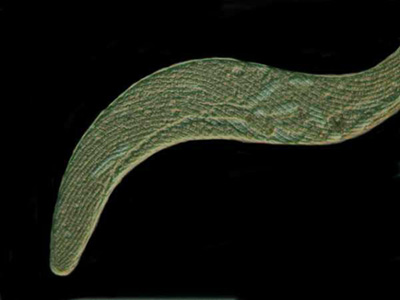
Below is a nice specimen of Spirostomum minus fully extended and, as you can see, it is long and quite slender.

The image below shows a specimen of what I believe to be S. intermedium. It was stained with a trichrome stain and clearly shows the nucleus beads.

The next image is a group of S. intermedium stained with Methyl Green Acetic. I have shifted the color balance slightly to produce a bit more contrast.
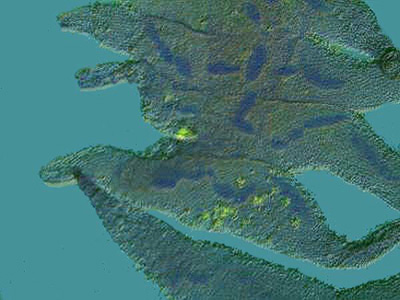
This specimen was also stained with Methyl Green Acetic and you can see not only the beaded nucleus, but the tiny fibrils that connect the beads.
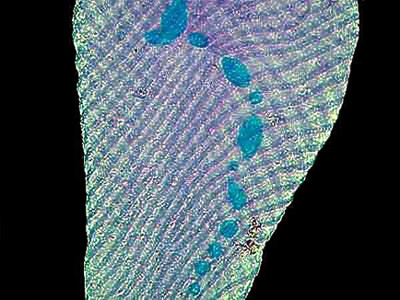
The final two images were taken of specimens treated with copper acetate. They were both taken with Nomarski DIC, but with a slight shift in the adjustment of the prism.
All comments to the author Richard Howey are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK