TOPICAL
TIPS
A small microfauna sediment sampler
Walter Dioni
Cancķn, Mťxico
A friend,
thinking that it could be helpful for my
researches, informed me of a small device of which he has had news,
although he
canít remember the source of the data. It turns out to be, functionally,
very
similar to my "Detachable-Neck
Flask" sampler.
(http://www.microscopy-uk.org.uk/mag/artmar05/wdextractor.html)
 I made a long and difficult search (because I didnít
know what the appropriate key words could be) but I found no one
reference
online.
I made a long and difficult search (because I didnít
know what the appropriate key words could be) but I found no one
reference
online.
But it's really useful, so I publish it, hoping that it serves others and, in addition, that someone can get an idea of who is the original author.
Note added March 2010: Many thanks to Paul Smith who read the article and the source, and communicated the bibliographic reference which is:Donald M Spoon, ďA new method for extracting and concentrating protozoa & metazoa from sediments"
Trans. Amer. Micros. Soc. 91 (4): 603-606 1972. Online Abstract at www.jstor.org/stable/3225492
I report here the procedures that were reported to me, but it is easy to see that these can
be
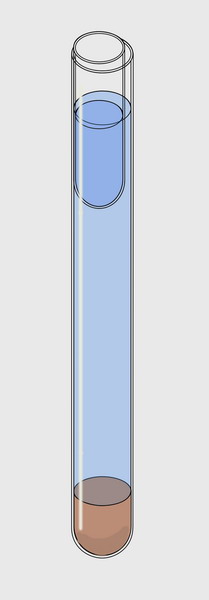
adapted to the needs of everyone. In a test tube, 17 mm internal diameter by about 15 cm
high, 2 cm of sediment were
placed at the bottom. Add water (preferably from the sampling site) to
4 cm of
the upper rim. And then slip inside, along the tube wall, another small
tube 5
cm long with an outer diameter
of 15 mm.
Injecting water
into the small tube (a dropper, or a hypodermic syringe could be
adequate) it sinks
until its content reaches the outside water level. A meniscus of water,
whose
greatest width is only 2 mm is so delimited. After 1 or 2 hours and for
the next
24 hours the micro-fauna which rises to the surface could be sampled,
taking
drops with a pipette. Of course, the water level drops by evaporation
(if not limited
and controlled using a humidity chamber) and by the withdrawal of
samples
itself. To maintain a suitable level of the meniscus, inject into the
small
tube a few drops of extra water. While the tube sinks, the outside
water level will
rise automatically. Simple, efficient and elegant donít you think?
To the right is
a schematic version of the instrument. The water is shown somewhat
below the
optimal level of work.
In the following
illustration (a version which my grandson Axel Quarchioni prepared in
C4D) a
suggested working assembly is displayed.
I didnít
receive instructions about this, but it seems logical to prepare an opaque
cardboard
cylindrical sleeve, such as that shown in the figure, to shield
the
sampler, leaving exposed only the top of the water column to stimulate
phototactic
responses.
Of course
dimensions are not critical. The really important thing is to maintain
a thin
meniscus, but one that supports the sampling with pipettes.
 As in the detachable
neck sampler, the collected
micro-fauna will be those organisms which are phototactic positive, geotactic
negative,
aerobic, and sensitive to the acidity of the medium. I imagine
that, as in my
instrument, the majority of the tigmotactic and geotactic negative
fauna will
die in the sediment**.
As in the detachable
neck sampler, the collected
micro-fauna will be those organisms which are phototactic positive, geotactic
negative,
aerobic, and sensitive to the acidity of the medium. I imagine
that, as in my
instrument, the majority of the tigmotactic and geotactic negative
fauna will
die in the sediment**.
The advantage of this little,
smart system, lies in the very small
sample volumes that can be processed, which in many cases is all that
is
available (think of small
ponds, tree holes, abandoned automobile tires, open
tin cans exposed to the weather, stagnant pools of rainwater from
bromeliads,
periphyton scrapings, small collections of filamentous algae, etc).
I know of
only one reader who has built one professional version of my
detachable-neck
flask sampler. If someone else has done experiments with that system,
or
tries this tiny version, I am interested in feedback and results.
** A suggestion, that
perhaps could work, would be to put over the sediment
a relatively thin disk (4or
5 mm) of medium or large pore polystyrene foam*, and then assemble the
instrument. The smallest and most mobile
micro-fauna
should cross the barrier to gather at the surface. Tigmotactic species
would invade
the new material, and could be sampled by removing and washing the sponge
in clean
water in a small capsule, at the end of the surface sampling time.
* This type of sponge is
common in Mexico, as it is used in medication bottles to prevent the
destructive movement of tablets.
Any piece of plastic foam of similar
size could serve.