The microscopical study of finely ground carbon suspended in uncovered alcohol
as first reported by Jan Ingenhousz in 1784:
Can replicating this simple experiment confirm that his own explanation of the particle motion was correct i.e. caused by evaporation currents and not the widespread modern reinterpretation as Brownian motion to give Ingenhousz priority over Brown?
by David Walker, UK
|
In Explorer browser, setting the custom print size to 60% prints out on 7 pages of A4.
|
Jan Ingenhousz FRS (also Ingen-Housz, IngenHousz or Ingen Housz, 1730-99) was a doctor and researcher whose scientific work included pioneering studies of photosynthesis (9,16,17). He was elected to a Fellow of the Royal Society in 1769.
He is also credited with the first report of the use of a coverslip (6) and to illustrate one of its main benefits he described a simple but effective experiment to demonstrate that uncovered liquids can evaporate quickly—the currents created imparted movement in all suspended inert objects that could be misconstrued as live organisms in motion when studied under the microscope.
A widespread modern reinterpretation of this work is that his explanation was incorrect but that he unknowingly observed what is now called Brownian motion over 40 years before Robert Brown in an inert subject.
Is this modern reinterpretation correct?
This article presents similar studies to those described by Jan Ingenhousz, includes embedded videos and compares these with true Brownian motion.
Image right: 'Jan Ingen-Housz'. Bust by F. Seifert. Vienna University. Frontispiece image from 'Jan-Ingenhousz. Sein Leben und sein Wirken als Naturforscher und Arzt' by Prof. Julius Wiesner, 1905. In the public domain at www.archive.org.
|

|
Introductory observations of popular demonstrations of Brownian motion
As a microscopy enthusiast I've always found demonstrations of Brownian motion fascinating. In liquids, typical methods to show this motion are in the fat globules of heavily diluted homogenised milk, a gamboge resin suspension in water or the carbon particles suspended in Indian ink. In the gas phase the Whitley Bay smoke cell is a popular method used in schools.
Only very modest microscopy equipment is required to show these demonstrations and have shared my own observations accompanied with videos in the two Micscape articles below.
Above: Brownian motion of fat globules suspended in very dilute homogenised whole milk.
Darkfield, objective 40X, eyepiece 10x, Optovar 1.25X. Fat globules ca. 0.5 to 3 µm in diameter.
From the author's November 1997 Micscape article 'Home in close-up: Studying Brownian motion'.
A 1080p HD extended video in darkfield is now available from this article page.
Above: Brownian motion in smoke particles suspended in air in a Whitley Bay smoke cell.
Darkfield, objective 6.3X. Particles are self luminous so shown as fine specks independent of size.
From the author's June 2009 article: Notes on demonstrating Brownian motion: The pros and cons of the Whitley Bay smoke cell compared with observing very dilute milk.
A 10x objective is suggested in the maker's instructions but the depth of field becomes quite small with smoke particles not being observed for long before moving out of focus. A 6.3x objective was better in this respect; the clip shows some motion by convection (along y axis in clip) in addition to Brownian
motion.
When preparing the most effective demonstrations it becomes very evident that two factors are key.
1) The particles must have a good proportion in the size range where Brownian motion can be observed. Taking suspended milk fat globules as an example, the motion becomes increasingly less pronounced for particles above ca. 5 microns and more pronounced as the globule sizes decrease below this.
2) All other possible contributions to particle motion must be minimised or preferably eliminated, such as convection currents caused by evaporation and/or by thermal gradients. At the typical magnifications required to see the motion, even small residual currents cause particle movements that can at best overlay and at worst mask the Brownian motion. A little residual motion by currents is tolerable for qualitative studies (and present in the Whitley Bay smoke cell) but must be entirely eliminated
for quantitative studies (12)
In the liquid phase, isolating just Brownian motion is readily achieved if use a larger cover slip with a thin liquid film and the slip completely sealed along its edges e.g. with Vaseline and left for some time. Currents by evaporation and convection are then almost totally absent. Such slides can be semi-permanent and inspected at will. If the slip isn't sealed, the currents caused by even the quite slow evaporation of liquid at the edges can be evident on particle studies.
The Whitley Bay smoke cell for gas phase studies can give a striking demonstration of the smoke particles 'dancing' against a black background but the typical cell design does suffer from convection currents which overlay the Brownian motion and also hampered by particles vertically moving through the narrow depth of field.
The study first reported by Jan Ingenhousz in 1784 and the modern reinterpretation of his work
The history behind the reported observations of what we now call Brownian motion have been extensively discussed—from possible reports prior to Robert Brown to Robert Brown's own work in 1827 (1-5,20). Later theoretical studies (notably by Einstein reported in 1905) and practical quantitative studies to confirm the theory (by Jean Perrin in 1908) provided indirect but firm evidence of the existence of atoms / molecules.
Robert Brown may not have been the first to observe the motion named after him—he used single lens microscopes for his studies which had been available since Hooke and Leeuwenhoek (19). Brown himself cited a number of possible observers in his addenda to his classic publication (3) and van der Pas made a careful summary of the early history (4,5,5a). However, Ingenhousz's work (which was
not cited by Brown but he may have been unaware
of it (5))
has attracted particular attention by van der Pas and more recent historians because Ingenhousz was describing some sort of motion in an inert substance thus removing the uncertainty as to whether the motions of live material being observed were contributing. The work of John Bywater published in 1824 (18) where he studied the motion of flour suspended in water has also been cited as a possible observation of Brownian motion in an inert substance prior to Brown—Brown cited this work but dismissed
as
an 'optical illusion' (3). Some recent workers regard it as a valid observation of Brownian motion (4) and Bywater's studies seems to merit a modern reassessment including experimental studies.
Many of the more recent writers (post late 1960s, e.g. 7,9,10,11,13-16,21) now attribute the first report of Brownian type motion in inert material to Jan Ingenhousz in his work of ca. 1784 where he studied finely ground carbon in uncovered alcohol (i.e. ethanol based). Thus predating Robert Brown's studies of 1827 by over 40 years. The details of the Ingenhousz study will be discussed in
more detail below but essentially he wanted
to demonstrate
the benefits of using a coverslip on liquid based subjects; a major one being to prevent rapid evaporation. The currents can cause excessive movement in suspended subjects to give a false impression that inert subjects may be living.
Given how important it is to minimise or ideally eliminate the slightest currents to truly observe Brownian motion and that Ingenhousz had designed an experiment to show rapid evaporation caused suspended particle movements, the justification of this widespread modern reinterpretation seems puzzling. To firmly assign Ingenhousz priority over Brown there needs to be firm evidence
that Ingenhousz incontrovertibly
observed what we now call Brownian motion in particles suspended in an uncovered low molecular weight alcohol.
This prompted me as a practical microscopist familiar with true Brownian motion to study Ingenhousz's original report, how it has been interpreted by various authors and to try and repeat a comparable study.
Jan Ingenhousz's reports of his finely ground carbon suspended in uncovered alcohol
Ingenhousz's study was described in a short five page article published in books compiling his other work. A paper by the late Peter van der Pas usefully provides the history of its publication and provides a full English translation (5). (Other English translations in whole or in part can be found in refs. 6,16,17). He notes that it was first included in a German publication of Ingenhousz's work in 1784 with the original French version published
in a later French edition in 1789.
I can't speak French or German so am indebted to this author for the translation
but the context and content does not seem to critically depend on the translation.
From the English translation, Ingenhousz very clearly discusses the potential problems associated with studying subjects in uncovered liquids under the microscope and the benefits of using a coverslip. These include reduction of evaporation and minimisation of optical artefacts
by creating a liquid film of uniform thickness. The first part of the article is quoted below sourced from ref. 5 which includes the short note on the charcoal/alcohol experiment, the second part includes suggested sources of suitably thin flat glass or mica and discusses further benefits of coverslip use. From this report, Ingenhousz is generally credited with this first use of a coverslip (as opposed to mounting microscopical subjects between mica discs which were already in common use) (6).
|
Extract from English translation source: P W van der Pas, 'The Discovery of the Brownian Motion', Scientiarum Historia, 1971, vol. 13, pp.27-35.
Primary French work and source for van der Pas's translation. 'Nouvelles Expériences et Observations Sur Divers Objets De
Physique' volume 2 by Jan Ingenhousz, 1789. pp.1-5.
[Pages 1-2] "REMARKS ON THE USE OF THE MICROSCOPE.
N.B. I have thought it appropriate to precede the following paper with these considerations which, although of no importance for those have frequently used the microscope, will at least be a guide to others.
I have often troubled my head about the problem to find a method to avoid the too rapid evaporation of a drop of water, or any other liquid, in which I wanted to observe the insects, and I know that other observers are plagued with the same problem. Even if one wishes to observe the shape and size of some of these corpuscules for even the short time during which such a droplet lasts in the focal point
of a microscope, one
must agree that, as long as the droplet lasts, the entire liquid and consequently everything which is contained in it, is kept in continuous motion by the evaporation, and that this motion can give the impression that some of these corpuscules are living, even if they have not the slightest life in them.
To see clearly how one can deceive one's mind on this point, if one is not careful; one has only to place a drop of alcohol in the focal point of a microscope and introduce a little finely ground charcoal therein, and one will see these corpuscules in a confused, continuous and violent motion as if they were animalcules which move rapidly around.
If the droplet is rather large, it has a convex surface which refracts the light more or less; if it is very small, it lasts hardly long enough to enable observing its contents at one's leisure."
... [page 4] Under these films, the vaporisation is so slow that a droplet which would evaporate in a few minutes, hardly vaporises in the course of hours. ...
[continues to page 5.]
|
Repeating Ingenhousz's charcoal in alcohol study
As the aim of Ingenhousz's original experiment was to show 'everything' in the liquid was in motion, it is arguably not critical for him to use extremely finely ground charcoal. Defining the evaporation rate at a stated room temperature for the low boiling point alcohol was also not key (Ingenhousz was in Vienna, Austria at the time his experiment was carried out and written up in the mid 1780s (7)). Although Ingenhousz does remark that in
his studies 'a droplet which would evaporate in a few minutes' (quote
above) indicating just how fast evaporation was occurring. A small drop of bioethanol on a clean slide in my room at 22 °C dried in about 1 minute. Droplets spreading less and at colder temperatures would last somewhat longer. However, to maximise the possibility of Brownian motion being observed in these
modern repeats, a
commercial hardwood fine charcoal was further finely ground to increase the concentration
of sub five micron sized particles. Ingenhousz used 'd'esprit-de-vin' i.e. spirit of wine or ethanol (b.pt. pure 78.4 °C), purity unknown. I used a bioethanol grade and have repeated similar experiments with isopropanol (b.pt. 82.6 °C) which is available in a pure reagent grade to hobbyists—the results are comparable to bioethanol.
As well as room temperature experiments, a cold stage was used to mimic a temperature more typical of a colder room. Initial trials showed that ethanol readily wets and rapidly spreads on a clean slide. This introduces large lateral movements into the particles before the liquid quickly dries. To what extent spreading occurred in Ingenhousz's study could vary dependent on alcohol purity
and nature of
the
glass surface. Constraining the ethanol in a ringed slide gave a reproducible drop thickness and was more favourable to observe Brownian motion
if it was possible.
Ingenhousz's report states that charcoal was 'introduced' into the alcohol. From trials, whether charcoal was added to alcohol or the reverse is not key—the alcohol instantly wets the charcoal which sinks to the slide base—particles that are in suspension show the same motion for each setup. For practical purposes for the embedded video recordings I added the alcohol to the charcoal.

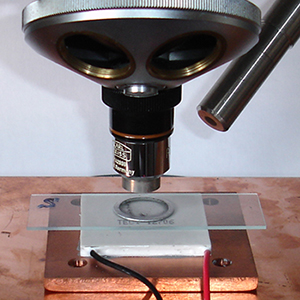
Above. Left. Materials used for the experiments. Bioethanol is a 95% pure grade of ethanol (remainder, higher alcohols and trace of denaturant) available to hobbyists like myself without the duty problems associated with a laboratory grade of pure ethanol.
Commercial grades of fine charcoal are still quite coarse, microscopically, so small amounts were thoroughly ground in an agate pestle and mortar.
To prevent extensive spreading of an unconstrained drop most studies constrained the ethanol in an aluminium ring 0.4 mm thick glued to the slide.
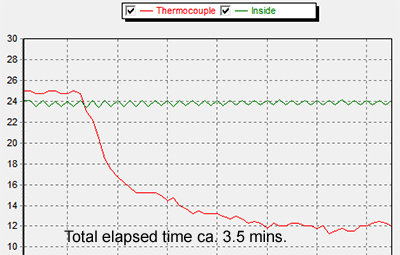
Middle: The speed of ethanol evaporation would be to some extent dependent on room temperature as well as other factors like drop size. My hobby room was typically 20-22 °C in August. To reduce the evaporation rate to that more typical of a colder room, a Peltier plate at 2.9V 0.8A was used to cool the alcohol and maintain it at ca. 12 °C. A light guide provided illumination. Colder temps. have been tried but below the dewpoint of ca. 9°C in my room causes condensation around the drop and may skew results because
of the hygroscopic nature of ethanol.
Particle motion does remain rapid due to evaporation at the lower temps.
Right: The ethanol temperature was monitored to equilibrium with a small unshrouded thermocouple junction and removed for the experiment. A separate article in the August 2015 issue of Micscape discusses the TEC1-12706 Peltier plate used (<£3 on eBay), the TEMPER1K4 USB thermocouple and cold stage designs tried for incident and transmitted illumination studies.
Above. Uncovered ethanol. Ethanol at room temperature ca. 22 °C. Horizontal field of view 3 mm. The motion of the charcoal
is exactly as Ingenhousz
describes, 'a confused, continuous and violent motion'. The motion is on such
a scale that only a Zeiss 2.5X
objective, total optical mag of 31X was used to video it (although the motion can be readily seen in the larger particles unaided with the eye, 10X hand lens or stereo). Particles and/or clumps of particles up to 100 µm in size are in motion. Given that an optical mag of typically 200 - 400x is required to see Brownian motion in sub ca. 5 µm particles in transmitted brightfield, the possibility of observing Brownian motion isolated from the massive currents under these or similar conditions with uncovered
ethanol are nil.
Above. Uncovered ethanol. Peltier cold stage, ethanol temp ca. 12 °C. Repeat of above at the lower temperature to mimic a colder
room
if this was the case
for Ingenhousz's study. The charcoal motion is still intense at a very low magnification and the exact temp. of his room is not key to determining whether he could have observed Brownian motion.
Above. Covered ethanol (horizontal field of view 3 mm) showing the edge of the coverslip. Zeiss 2.5X objective. The benefits of using a
coverslip as described by Ingenhousz are evident. Evaporation and the associated particle movement is now restricted to the exposed alcohol at the edges. This edge evaporation does still cause a still finite continuous liquid movement towards the edges, and causes suspended finer particles to move. Modern studies of Brownian movement in the liquid phase, seal the coverslip edges for this reason (12).
Could Jan Ingenhousz have observed Brownian motion?
To date I have not been able to find any reference to the type of microscope used by Ingenhousz and its optical performance (any information welcomed). But high powered single lens microscopes had been available since Leeuwenhoek / Hooke (19) and could have shown the motion. My own studies of the finely ground charcoal show that if the sample is covered with a coverslip it is possible to observe Brownian motion. In ethanol the motion of the finest particles is overlain by the evaporative currents
towards
the edges of the finest particles, in water less so (see video below) but the modern worker is aware of and looking for the underlying Brownian motion. Although despite very finely grinding my own sample of charcoal, relatively few particles are in the correct size range. However, as discussed and illustrated in this article, Ingenhousz's experiment as described in uncovered ethanol was not capable of isolating Brownian motion from the dominant motion caused by evaporation.
Above. Finely ground charcoal in ethanol with coverslip, higher magnification. Room temp. 22°C. Zeiss 40X NA0.75 Neofluar objective, total optical mag 500X. Video frame
field of view 0.19 mm. Unlike for uncovered ethanol, most larger particle motion is stopped. Finer
particles small enough to show Brownian motion (< ca. 5 microns) are present
in small quantities. e.g. see particle third in from left and third up, just to the right
of the triangular particle. Although most finer particles are moved by currents caused by ethanol evaporating at
the edge of the coverslip.
Comments on the modern reinterpretation
The earliest reference found to date which interprets Ingenhousz's observations as Brownian motion is a short note at the end of a paper entitled 'The Early History of the Brownian Motion' presented by the late Peter van der Pas at an international congress on the history of the sciences in 1968 (4). The same author published a paper entitled 'The Discovery of the Brownian Motion' devoted to Ingenhousz's studies in 1971 (5) and was appended
by a complete English translation
of Ingenhousz's
relevant essay (quoted in part above). The entry for 'Ingen-Housz, Jan' in the 'Dictionary of Scientific Biography', the leading resource for biographies of scientists, also states Ingenhousz's priority (7).
Presented below is a summary of the evidence that Ingenhousz's original interpretation was correct and that he did not unwittingly make the first observation of Brownian motion in an inert material.
-
Primary evidence from Ingenhousz's essay
-
The context of his article is set in his introductory 'N.B.' Nota bene, i.e. describing a problem with uncovered liquids that was 'of no importance' to experienced microscopists implying that it was a familiar phenomenon rather than reporting a previously unobserved motion independent of evaporation effects.
-
He clearly explained that the aim of the experiment was to demonstrate the benefits of a cover slip—without one rapid evaporation from an uncovered liquid causes 'everything which is contained in it' to be in 'continuous motion'.
-
To emphasize the point he suggest sprinkling finely ground carbon into a low boiling point alcohol (ethanol), not water.
-
He described this motion as 'violent' (original French = English equivalent) which does accurately describe the up to macroscopic scale motion of all sized particles if repeated but it does not describe Brownian motion, which is jiggling of ca. sub 4-5 µm particles on a microscopic scale.
-
-
Evidence from repeating comparable experiments
-
Particles up to 25X larger than those in which would exhibit Brownian motion are in motion cause by the evaporation— the lowest power of a compound microscope (2.5X objective) is required to keep them in the field of view cf the 40X objective typically required to observe the in the motion in the liquid phase.
-
Ingenhousz's room temperature for his studies is unknown, but evaporation remains by far the dominant motion even when mimicking a potentially colder room.
-
Evidence lacking, to incontrovertibly assign priority of observing Brownian motion to Ingenhousz in favour of Brown
-
The evaporative motion is so marked, even in particles or clumps of particles up to 100 µm in size from my own studies, that the particles do not have to contain a significant fraction in the size range to observe Brownian motion, typically less than 4 µm. He does not define and does not need to state what he means by 'finely ground'—but it seems vital to be certain that he observed Brownian motion even if he had minimised
evaporative effects.
This is in contrast to Brown's work. The pollen grain sizes of Clarkia pulchella are measurable as are the fine particles within the pollen grains that when released from burst grains in water were the basis of Brown's first study. He later studied fine particles in a variety of inert materials to confirm it was not associated just with living organisms. He also did careful studies to eliminate all possibility of evaporation with an elegant experiment where he suspended water droplets containing
suitable particles within an immiscible oil.
-
If Ingenhousz had repeated the study using an uncovered liquid less prone to very rapid evaporation, e.g. water rather than alcohol, water does not readily wet carbon and many smaller particles would have floated on the surface. This is a system notoriously prone to another outside agent—vibration—and which could potentially mask the observation of Brownian
motion. The system is so sensitive
to vibration, sprinkling fine dust e.g. carbon onto the surface of an uncovered bulging drop of water has been suggested as a way of assessing a microscope-camera setup for its stability to vibration. Loveland in his authoritative classic text 'Photomicrography. A Treatise' (8) remarks on how 'exceedingly delicate' this test is and cites Shillaber who remarked it was 'too delicate'. My 20 kg plus Zeiss Photomicroscope III sits on a very study desk for photography
up to the very highest powers. Studying a microscope slide with a ring cell in which fine particles are sprinkled onto a bulging water drop can pick up the vibration of the lightest tap of my little finger on the desk.
Concluding comments
Accurately assessing some practical aspects of historical microscopical work in the context of the time rather than in the light of our modern knowledge can be difficult, especially where one or more of factors such as the microscope, optics performance, experimental conditions and experimenter's skills can be key. But in this example, these factors are not key. Repeating the
experiment under the most favourable conditions demonstrates that the extensive evaporation movements completely overwhelms the chance of observing the Brownian motion in uncovered alcohol.
A modern worker of course also has the privilege of knowing that there is an underlying movement to watch out
for in suitably fine particles suspended in liquids. If Ingenhousz did suspect such a movement, such an experienced researcher would likely have repeated the experiment using two methods to minimise evaporation: 1) using water not alcohol and 2) using the very device he was advocating the benefits of and which he is credited with inventing—the coverslip.
It could be argued that the modern reinterpretation of Jan Ingenhousz's study where it is frequently stated in various forms that he overlooked and/or didn't follow up his observation of what we now call Brownian motion in an inert material does a disservice to his skills as an astute experimenter and observer. He was one of the finest researchers of his day. The evidence seems compelling
that he
knew exactly what he wanted to show, describes a simple experiment
to show it and correctly interprets the results—which can be confirmed by modern comparable experiments using the most basic of microscopes, either low power compound or a stereo.
Two extensive biographies have been recently published on Jan Ingenhousz and his work—both admirably fulfilling their aims that this man's varied work receives much greater recognition. It's interesting to note that the respective authors take opposing views on attributing Ingenhousz's charcoal in uncovered alcohol study as Brownian motion. Magiels in his 'From Sunlight to Insight'
2010 (16) in a section
entitled 'And
in the mean time, some IngenHousz Motion', adopts the current modern reinterpretation that Ingenhousz did unwittingly observe Brownian motion. Beale and Beale in their 'Echoes of Ingen Housz' 2011 (17) which I obtained a copy of after near completion of my own studies are not convinced by this modern reinterpretation; after citing both van der Pas's 1968 and 1971 papers they remark:
'The justification is, however far less convincing than it is for Ingen Housz having devised the cover slip and is almost certainly an over-enthusiastic conjecture.' Beale and Beale 2011 (17).
Beale and Beale have succintly summarised what regrettably seems to have occurred (also see note in ref. 17 below). But other than these authors, of all the reports in scientific papers, books on Brownian motion and authoritative biographies inspected to date where both Ingenhousz and Brownian motion are discussed, Ingenhousz's study is now either interpreted as having priority over
Brown
at observing Brownian motion in an
inert material or in at least one case (14) accepting Ingenhousz did observe first but retaining Brown as the discoverer because Ingenhousz did not follow up.
I reach a similar conclusion to Beale and Beale after inspecting the published English translations of the primary source (5, 6, 16, 17), together with my own experiences of demonstrating Brownian motion in both the liquid and gas phase and by performing simple experiments that are comparable to that of Ingenhousz as described above.
The otherwise widespread attribution of Ingenhousz's priority at observing the motion in an inert material over 40 years before Brown certainly seems to merit a reassessment by historians.
Comments to the author David Walker are welcomed.
The author is a microscopy enthusiast with a particular interest in demonstrating Brownian motion. A current project is studying the motion in gamboge to appreciate the
careful quantitative experimental studies by Jean Perrin that confirmed Einstein's theoretical studies.
References consulted, selected quotes and supporting notes (links where provided are to full copies of papers if freely available on the Internet).
1) Robert Brown, selected extracts and comments compiled by J Ramsbottom, in 'Centenary of Robert Brown's discovery of the nucleus exhibit at Natural History Museum, The Journal of Botany,
132, vol. LXX. Quotes in full, notes by Brown headed 'June 12 1827 [Clarkia]' where he makes his earliest report of the motion of
particles originating from within pollen grains. A reference previously unknown to me and I am indebted to Pearle et al's own citation ref. 21 (10).
2) Robert Brown, 'A brief account of microscopical observations made in the months of June, July, and August, 1827, on the particles contained in the pollen of plants; and the existence of active molecules in organic and inorganic bodies.' Dated July 30th, 1828.
Privately published originally and later published in various journals. This and reference (3) are available as scans of the original private publication in a single pdf hosted by the New York Botanical Gardens website. Both references have also been transcribed into a web page on the Wikisources website with internal relevant links.
3) Robert Brown, 'Additional remarks on active molecules', dated July 28th 1829. See above for public domain resource.
4) P W van der Pas, 'The Early History of the Brownian Motion', XIIe Congrès International d'Histoire des Sciences, Paris 1968, Actes Tome VIII, Histoire des Sciences Naturelles et de la Biologie, pp.143-158. Publisher Albert Blanchard, Paris, 1971. French/English papers, the van der Pas paper is in English.
5) P W van der Pas, 'The Discovery of the Brownian Motion', Scientiarum Historia, 1971, vol. 13, pp.27-35.
Peter van der Pas was born in
The Netherlands where Ingenhousz was also born.
Quote p.31 (his capitals): 'While discussing the problems due to evaporation of a liquid under the microscope, INGEN-HOUSZ mentions his observation of the Brownian motion:'
Later after quoting Ingenhousz: 'Here the two elements of the Brownian motion, the rapid motion of the corpuscules and the fact that the motion is also shown by non-living material are presented by INGEN-HOUSZ in only two sentences, which are buried in the presentation of quite another subject. Clearly, INGEN-HOUSZ did not realize the importance of this observation, if he had done so, he would have elaborated on the subject and perhaps even conjectured about the cause of the phenomenon.'
Later p.32: 'It will be clear that INGEN-HOUSZ has an earlier claim to the discovery of the Brownian motion than ROBERT BROWN himself.'
5a) Allison Fife, 'Page by Page: Reconstructing an Intellectual's Drive to Collect', Utah Historical Review, July 2013, vol.
3, p.215ff.
A detailed and fascinating account of aspects of Peter van der Pas's life and work. In particular as a noted bibliophile and who founded a library based on his own collection.
6) H E Hoff, 'Jan Ingen-Housz and the Cover-Slip', Bulletin of the History of Medicine, 1962, vol. 36, pp.365-368.
7) P W van der Pas, 'Ingen-Housz, Jan', entry in the 'Dictionary of Scientific Biography', Volume VII, pub. Charles Scribner's Sons, New York, 1973.
Quote from p.13: 'But perhaps the most important consequence of his algae research was his discovery and correct interpretation of Brownian motion' and later 'Although Ingen-Housz has not been properly credited with this discovery, ...'.'
8) R P Loveland, 'Photomicrography. A Comprehensive Treatise', volume 1, p.99-100, publisher John Wiley & Sons, Inc, New York, 1970.
Loveland cites Shillaber's* suggestion to sprinkle e.g. carbon dust onto a bulging water drop in a cell on a microscope slide. Loveland notes, quote: 'This test is exceedingly delicate; Shillaber believes it is too delicate.' (*Loveland's ref. C B Shillaber, 'Photomicrography', Wiley, New York, 1944.)
9) P Smit, 'Jan Ingen-Housz (1730-1799): Some New Evidence About His Life and Work', Janus, 1980, pp.125-139.
Citing van der Pas's 1968 paper (40) he remarks:
Quote: '... his researches nevertheless led him to the discovery of swarm pores, to the introduction of cover-glasses in microscopic investigations and he described exactly the Brownian movement long before Brown himself. He made this last mentioned discovery with fine carbon particles suspended in alcohol in which he investigated the same kind of irregular movements as he already knew of from infusoria swimming in water.'
10) P Pearle, K Bart, D Bilderback, B Collett, D Newman and S Samuels, 'What Brown Saw and You Can Too', American Journal of Physics, 2010, vol. 78, pp. 1278-89.
This superb paper includes a detailed description on how to repeat Brown's studies using Clarkia pollen and instructions on how to make a single lens microscope 'with a magnification comparable to Brown's'. Their ref. [68] cites van der Pas's 1971 study. In notes accompanying this reference the authors remark:
Quote: 'He [van der Pas] wrote this paper to draw attention to a rather throw-away paragraph in a paper in 1784 by Jan Ingenhousz, thereby intimating Ingenhousz's priority over Brown. The purpose of Ingenhousz's paper was to introduce the idea of a transparent cover slip in microscopy to prevent water evaporation. Unlike others who had seen Brownian motion before Brown, but attributed it to life, Ingenhousz clearly asserted in this paragraph that nonliving matter underwent the [Brownian] motion, but he did
no systematic investigation.'
11) M F Shlesinger, 'Physics in the Noise', Nature, 2001, vol. 41, 7th June, p.641.
Quote: Summarising the history of Brownian motion. 'In 1785, Jan Ingenhauz [sic] had placed charcoal powder on an alcohol film and saw the grains moved randomly.'
12) M A Catipovic, P M Tyler, J G Trapani and A R Carter, 'Improving the Quantification of Brownian Motion', American Journal of Physics, 2013, July, vol. 81(7), pp. 485-491.
13) P Hänggi, F Marchesoni and F Nori, 'Brownian Motors', Ann. Phys, 2005, vol. 14, nos. 1-3, pp. 51-70.
Quote p.1. 'Einstein seemingly was
unaware of the earliest observations of Brownian motion under a microscope: namely, the work of the
Dutch physician Jan Ingen-Housz [4] [citing Ingenhousz's 1784 German and 1789 French article and van der Pas's English translation 1971], who detected, probably first, Brownian motion of finely ground
charcoal particles in a suspension at the focal point of a microscope, and the detailed studies by the renown
botanist Robert Brown [5]. In clear contrast to Robert Brown, who performed a series of experiments,
Ingen-Housz provided a quite incorrect physical explanation of his observations by ascribing the effect to
the evaporation of the suspension fluid.'
14) R M Mazo, 'Brownian Motion. Fluctuation, Dynamics and Applications', Clarendon Press, Oxford, 2002, p. 3. Amazon UK 'Look Inside' view provides access to the relevant page.
Quote p.3: [After citing the relevant part of an English translation of Ingenhousz's article]. 'On the basis of these few lines, Van der Pas (1971) has argued that Ingen-Housz should be accorded priority in the discovery of Brownian motion. However, although Ingen-Housz doubtless observed the motion, he did not follow up his investigation of it, as did Brown. His ascription of the motion to evaporation was, as Brown showed in a simple experiment, quite wrong. Robert Brown still
deserves pride of place with respect to the discovery of the motion now named after him.'
15) D J Mabberley, 'Jupiter Botanicus. Robert Brown of the British Museum', publisher J Cramer, Braunschweig, 1985, p. 268-274. p.272-273.
Quote p.272-3: citing van der Pas 1971 '... the movement, which now bears his [Brown's] name, was first noted by Ingen-Housz in 1784. It must be admitted, however, that Ingen-Housz's remark is buried in a paper on another topic: indeed it was overlooked
until 1971, a technical 'first' with little importance in the history of science. Further, Ingen-Housz did not realise its importance or investigate it in any scientific way. Brown's paper is a tour de force ... .'
16) G Magiels, 'From Sunlight to Insight'. Jan IngenHousz, the discovery of photosynthesis and science in the light of ecology', publisher VUBPress Brussels University Press 2010, chapter 4 section headed 'And in the mean time, some IngenHousz Motion'. pp. 178-182. Includes extensive French quotes from IngenHousz's essay and own English translation.
Quote from p.179: 'This [the charcoal in uncovered alcohol experiment] is particularly interesting because here he not only gave the first description ever of the Brownian motion, but also provided a description of the use of a cover slip (also a première).'
Quote from p.181 after earlier citing van der Pas 1968: 'Clearly IngenHousz did not realise the importance of his observations and techniques. It shows the pragmatism of the man.' and later
'IngenHousz has been forgotten for many reasons. In this particular case, it did not help that he did not see it himself as an important discovery. Looking at the way he described it, he also was not interested in a physical explanation for what happened under his lenses.'
17) N and E Beale, 'Echoes of Ingen Housz. The long lost story of the genius who rescued the Habsburgs from smallpox and became the father of photosynthesis.', publisher The Hobnob Press, Salisbury, UK, 2011, pp. 344-346.
Quote p.344: 'Another claim has been made for Ingen Housz in the important field of microscopy. It has been argued that he was the first person to observe what has become known as 'Brownian Motion'. And later after citing both van der Pas' 1968 and 1971 papers (4,5) remark: 'The justification is, however far less convincing than it is for Ingen Housz having devised the cover slip and is almost certainly an over-enthusiastic conjecture'.
These authors also discuss a later part of Ingenhousz's article not quoted above on his described benefits of the use of a coverslip which they perceptively note on p.345 offers 'clinching evidence that Ingenhousz was not observing Brownian motion.'
18) John Bywater, 'Physiological Fragments: To which are added Supplementary Observations to show that vital and chemical energies are of the same nature, and both derived from solar light.'
London 1824, p.40ff.
19) Brian J Ford, 'Single Lens. The Story of the Simple Microscope', publisher Heinemann, London 1985.
20) Brian J Ford, 'Brownian movement in Clarkia pollen: A reprise of the first observations', The Microscope, 40 (4): 235-241, 1992.
21) Bertrand Duplantier, 'Brownian Motion, "Diverse an Undulating"', "Einstein, 1905-2005". Poincaré Seminar 2005, Th. Damour, O. Darrigol,
B. Duplantier and V. Rivasseau, Editors, pp. 201-293 (Birkhäuser Verlag,
Basel, 2006) 'Expanded and updated version (13 May 2007)'. A superb paper that gives a thorough discussion of the history of Brownian motion studies—both experimental and the development of mathematical theory.
Quote pp.206-207: Citing Mazo (14), van der Pas (5) and in part Ingenhousz's article from (5), notes, ''However, although Ingen-Housz doubtless observed the motion, he ascribed it to evaporation and did not follow up his observation with any investigation of it."
Later on pp. 209-210 - presents a discussion of Brown's method to reduce evaporation with his water drop in oil experiments, quoting Brown who was aware that evaporation is an important and potentially conflicting source of motion.
Revision history.
August 13th 2015. First published.
August 16th 2015. Added Ingenhousz's quote on alcohol droplets lasting only a few minutes and comment on typical evaporation rate in present author's own room.
August 27th 2015. Added ref. 21 with quotes and note.
©
Microscopy UK or their contributors.
Published
in the August 2015 edition of Micscape.
Please
report any Web problems or offer general comments to
the
Micscape
Editor
.
Micscape
is the on-line monthly magazine of the Microscopy UK web
site at
Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
.



