In my previous article, various forms of fixation and types of fixative were discussed. Many interested readers responded and posed questions about formulae, both of fixatives and stain solutions I used, as reflected in my photomicrographs. As a general response to enquiries, a pdf document containing technical info is attached to this article.There is a special group of stains, the vital stains, which are capable of penetrating the cell membrane of living cells/organisms since they possess a lipophilic property. Some of them bind specific sites like mitochondria in the cytoplasm in vivo, hence vital stain. They are normally used in very dilute concentrations to avoid any interference between the living state and the stain itself. Ultimately, each vital stain may lead to cell deterioration especially in higher concentration. A wonderful account about vital stains written by Richard L. Howey can be found in the Micscape Library. The vital stains entering into the cell or tissue cause visible changes. If cells are fixed and thus killed, their membrane permeability increases; many agents can enter the cells easily and many structural molecules can leave cells and tissues as well. Some fixatives cause a rapid and sometimes excessive loss of such structural components. Consequently, fixed cells or tissues undergo irreversible changes during fixation.
If you apply a method of chemical fixation and your fixative contains alcohol, you have to expect visible deformities due to severe shrinkage caused by cell dehydration. A 10 to 30% decrease of overall size is not unusual! If your fixative contains heavy metal ions such as zinc (Zn2+) or mercury (Hg2+) they may coagulate and precipitate proteins in granular form. Tannin, arsenic, detergents and acids may be components of a fixative (especially in the formulae of the good old days!) and their deleterious effects depend on the concentration and the duration of the fixation. Nevertheless, many still prove to be very useful and even unique in some specific conditions. For example, osmic acid (OsO4) is a perfect fixative for fatty substances and is preferred as a secondary fixative of samples for electron microscopy. Unfortunately osmic acid and some other fixatives are extremely toxic and should only be accessible and safely used in professional laboratories.
For chemical fixation there must be a compromise. A solution with less negative effects on cells and tissues can be achieved by using aldehydes such as formaldehyde and/or glutardialdehyde. An aqueous 4% formaldehyde solution is an almost universal fixative. If substituted with glutardialdehyde, it works as a useful fixative for both light- and electron microscopy purposes (see the attachment). Of course, pH and osmolarity are aspects of the fixative for which researchers must refine their working solution.
I want to draw your attention to the effect of temperature on fixation. The higher the temperature, the more rapidly occur the chemical binding processes and penetration of the fixative. But we have to take into account that higher temperatures may cause higher intrinsic enzymatic activity within the cells/tissues which damages the microscopical structures. Low temperatures between 0°C - 4°C is therefore preferable for specific purposes. In scientific applications, the chemical composition of the fixatives, the buffers they contain, the pH and osmolarity determine the results desired. For us, as amateur microscopists, I should say fixation at ambient temperature would suffice.
In the last ten years, a new way of fixation namely 'microwaving' becomes more attractive. Nowadays, microwave ovens usually belong in modern kitchens. We have a good chance to fix our material by using such household MW-ovens. (See safety footnote, and follow the manufacturers precautions). I have thoroughly experimented with fixation and staining using MW-ovens for more than 10 years on a wide range of materials and samples. It does work simply IF YOU KNOW what are you doing with that machine.
Use large glass bottles to irradiate fluids. Do not fill up but keep them half empty. It is essential to use much lower power than usual or even the lowest power setting (usually 800 Watt is the highest for household MW-ovens, and 80 watt the lowest) for 15 minutes. But do not cook it! If your MW-oven has a temperature probe, set it up to 30°C for automatic control. I use such an MW-oven and glass boxes of 200 ml in volume. It is then filled with a fixative solution about 100 ml. The sample to fix is put in it and microwaved for 1 hour at the lowest power setting or temp. control set at 30°C. In all my work over the last 10 years, I have exclusively applied this form of chemical fixation (see all micrographs presented in my previous articles in the Micscape) both for light- and electron microscopic fixation. Below, I give two examples of TEM images of a fungal hypha primarily fixed using a mixture of formaldehyde and glutardialdehyde (see the pdf attachment) in a temperature controlled microwave oven, and thereafter secondarily fixed using a ferro-osmium complex at room temperature for 4 hours (without microwaving). If your MW-oven does not have a low energy setting (e.g 20% of full power) please DO NOT carry out fixation trials! Microwaves produce a tremendous amount of heat within many substances and within a few seconds of start up! Once again, consult the manufacturers instructions for technical and safety reasons.
Microwaving biological material produces primarily molecular stabilization and then greatly increases the transportation of fixative molecules deep within the sample. Remember, in our usage, we DO NOT intend to cook the samples! I am sure, most amateur microscopists will discover his/her way of applications with the MW-ovens since they are readily available to us.
Safety footnote: A dedicated MW-oven with low power or temperature control features should be sourced and used in an appropriate environment for safely handling chemicals. On no account should a MW-oven used for chemical treatments also be used for food preparation.
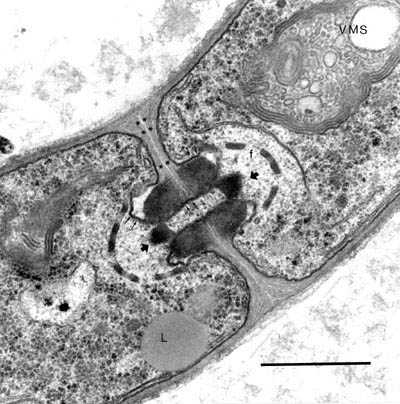
Fig. 1. TEM image of a fungal hypha of Agaricus bisporus. Hypha showing portions of two segments (cells) which are incompletely separated by the so-called dolipore septum, visible as an elaborate structure at the centre. A fenestrated sieve membrane and two plugs on both side of the septum are apparent (arrow heads). These plugs are heavily fixed and stained by osmium molecules of the secondary fixative. A round organelle marked with letter L is a vacuole filled with fatty (osmiophilic) susbstace. The scale bar is 2 micrometer.
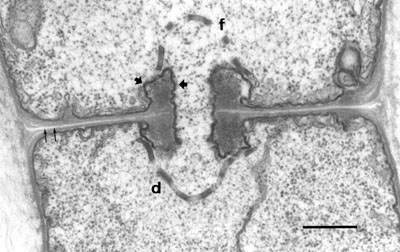
Fig. 2. TEM image of a fungal hypha of Agaricus bisporus. The details of the membranes around the dolipore septum and its fenestrae are shown. The scale bar is 1 micrometer.
Comments to the author M. Halit Umar are welcomed.
~~~~~ The author's affiliation:
MES Laboratories, P.O. Box: 6042,
5960 AA HORST, The Netherlands. Phone: (0031) 77 464 7575 , FAX: (0031) 77 464 1567